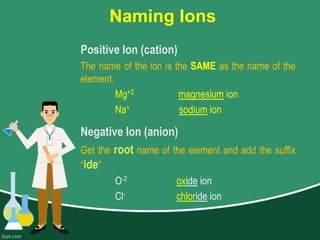
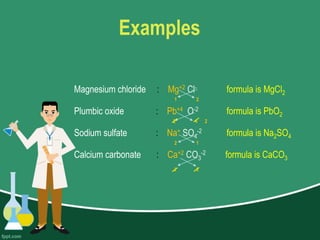
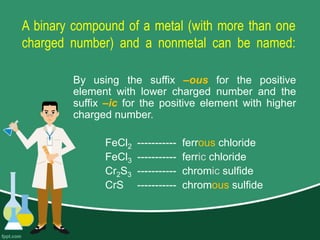
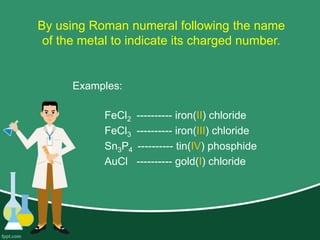
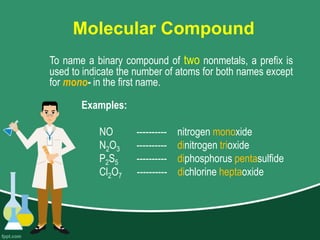
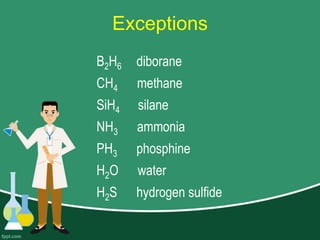
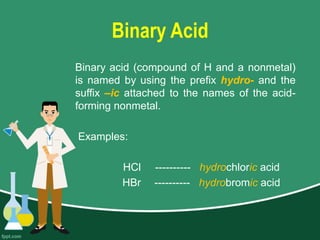
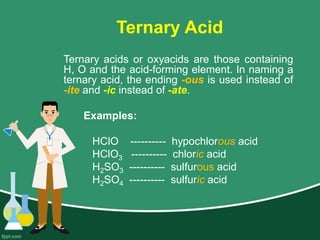
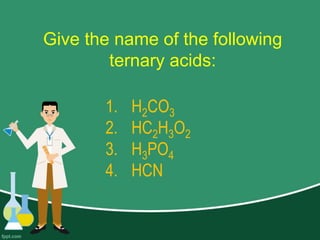
This document provides information on naming ions and compounds. It discusses rules for naming positive and negative ions, as well as binary ionic compounds, binary molecular compounds, exceptions, binary acids, ternary compounds, and ternary acids. Examples are given for each type of compound. The objectives are for learners to be able to name compounds from formulas and write formulas from names. Review questions and an assignment are included at the end.