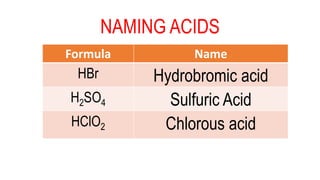
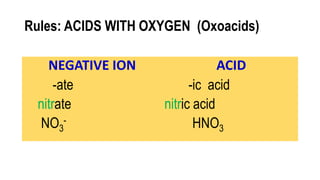
This document discusses naming acids based on their chemical structure. An acid is a chemical species that donates protons or hydrogen ions. The name of an acid is based on the name of the negative ion that is part of the acid. For acids without oxygen, the suffix is replaced with "hydro" and "ic acid". For acids with oxygen, oxoacids, the suffixes are "-ous acid" for -ite ions and "-ic acid" for -ate ions, with few exceptions like phosphoric and sulfuric acids. Examples are provided to demonstrate the naming conventions.