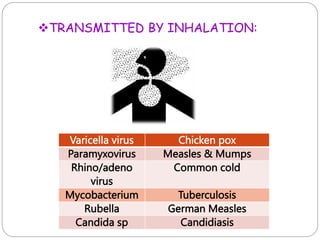
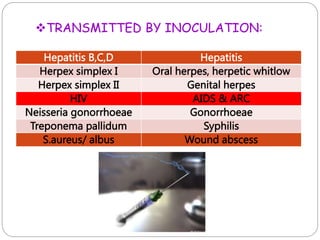
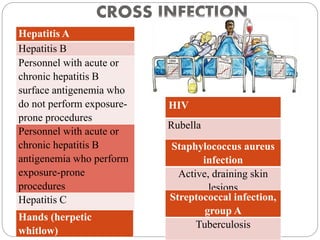
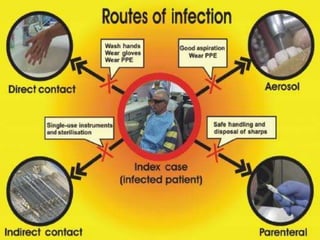
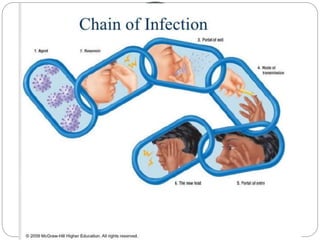
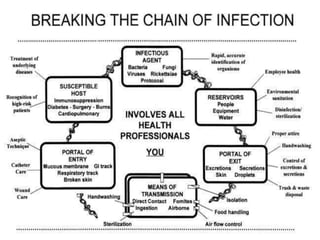
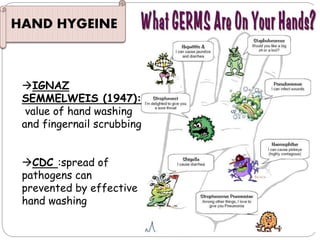
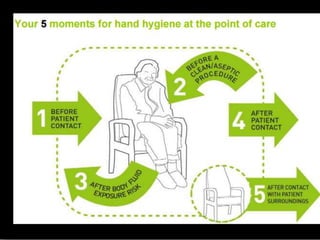
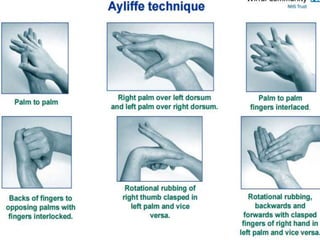
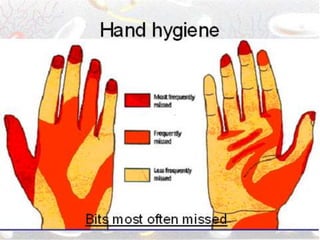
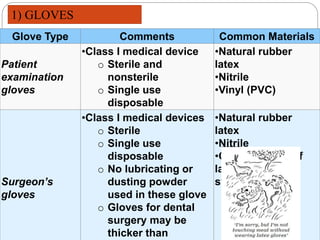
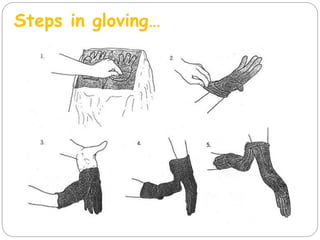
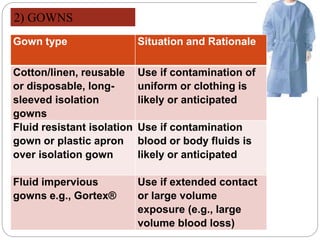
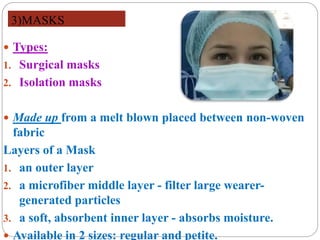
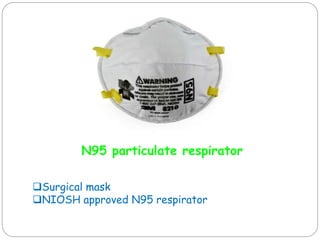
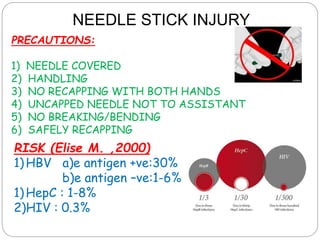
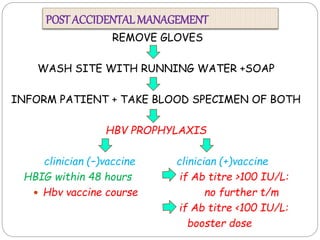
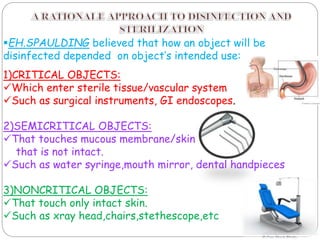
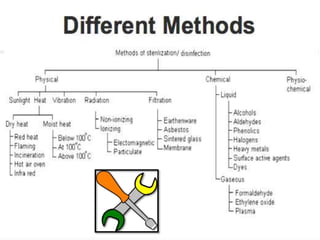
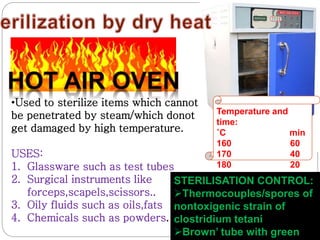
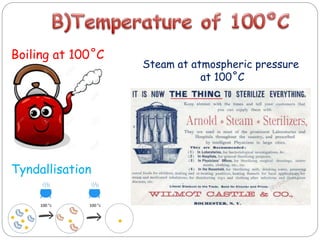
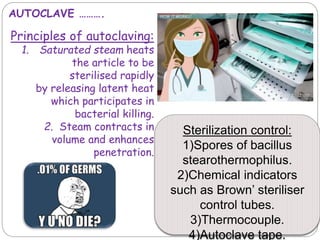
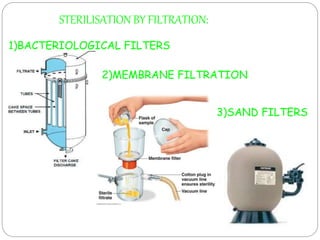
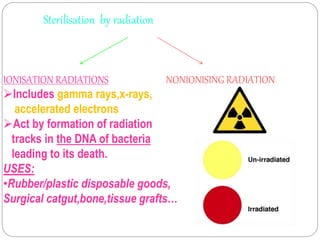
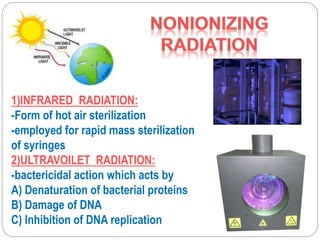
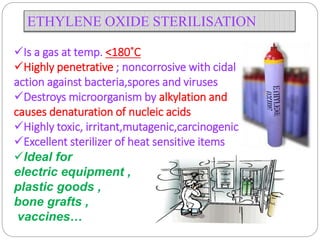
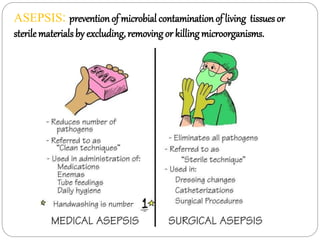
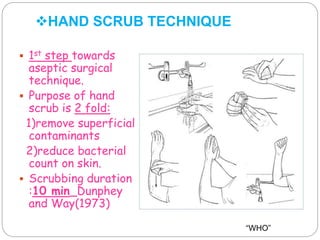
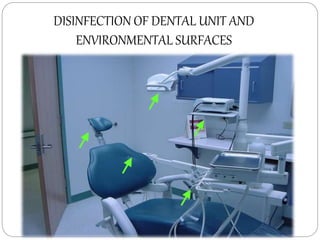
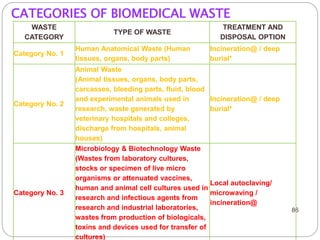
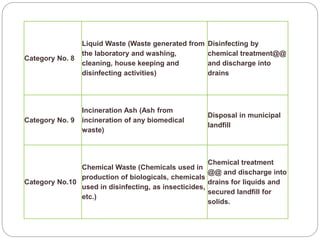
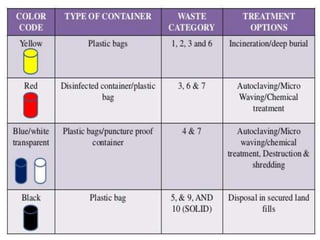
The document discusses infection control procedures in dentistry. It covers topics like hand hygiene, personal protective equipment (PPE), sterilization, cleaning, disinfection, asepsis, operatory room procedures, and waste management. Proper infection control is important to prevent the transmission of infectious diseases between patients and dental staff. The key strategies for infection control include hand hygiene, use of PPE like gloves, gowns and masks, sterilization of instruments, cleaning, disinfection and proper waste disposal. Regulatory agencies develop guidelines to maintain minimum health and safety standards in dental facilities.