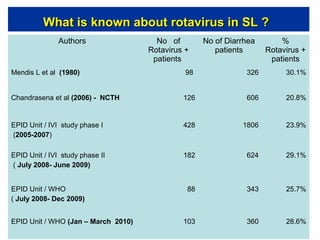
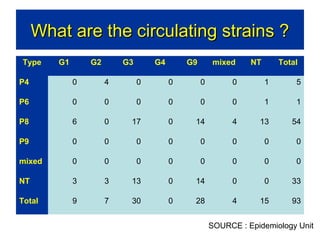
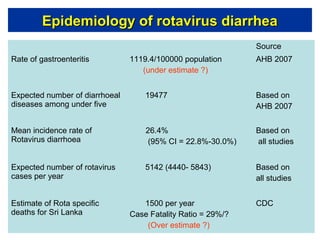
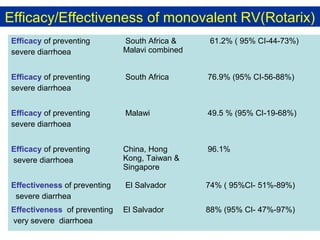
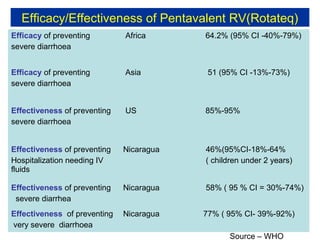
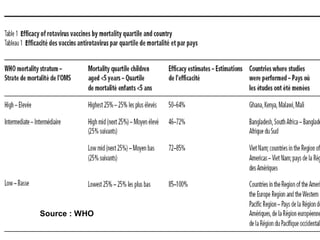
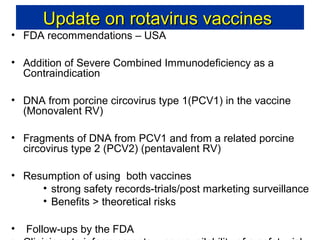
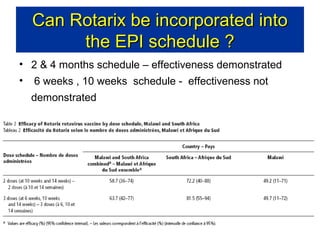
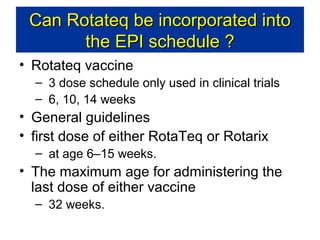
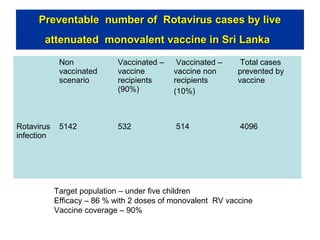
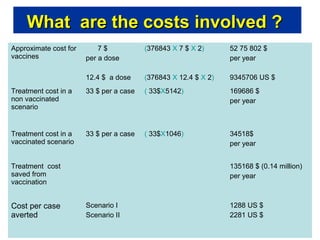
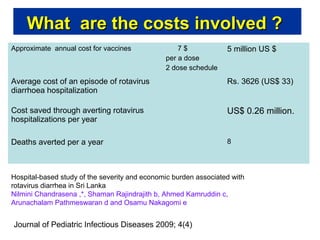
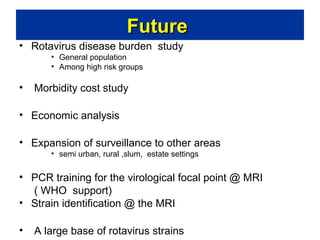
Rotavirus is a major cause of diarrhea among children under 5 years old in Sri Lanka. Surveillance data from 2005-2010 showed that rotavirus accounted for 20-30% of diarrhea cases. The dominant circulating strains were G3 and G9. It was estimated that rotavirus causes over 5,000 cases and 1,500 deaths per year nationally. Two rotavirus vaccines (Rotarix and RotaTeq) have shown efficacy of 61-96% in other countries against severe rotavirus diarrhea. Introduction of the vaccine in Sri Lanka could prevent over 4,000 cases annually and save $135,000 in treatment costs. Further disease burden and economic studies were recommended to inform the decision on vaccine introduction.