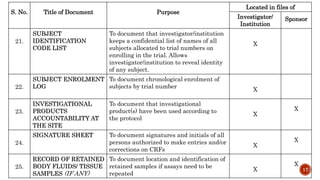
This document provides guidance on essential documents that should be maintained for clinical trials. It is divided into three sections based on the stage of the trial: before, during, and after. The document lists important documents such as the investigator's brochure, signed protocol, IRB approval, CRFs, monitoring reports, and financial agreements. It explains that maintaining proper documentation allows evaluation of trial conduct and data quality in compliance with regulations.