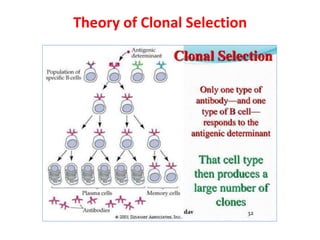
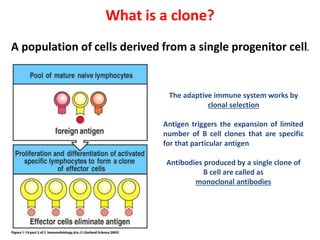
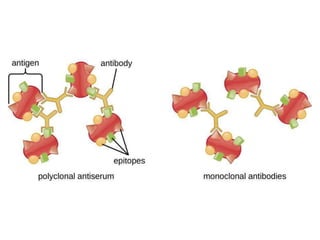
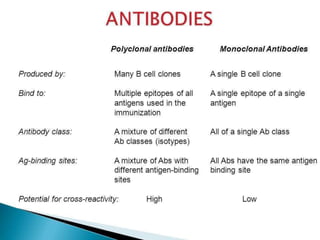
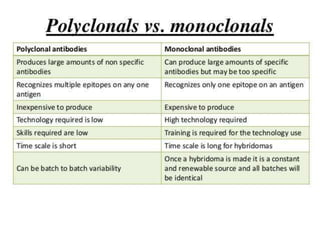
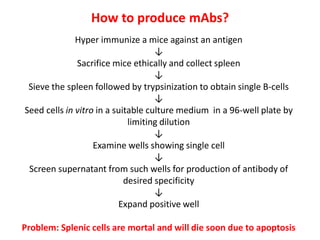
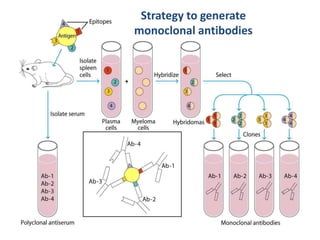
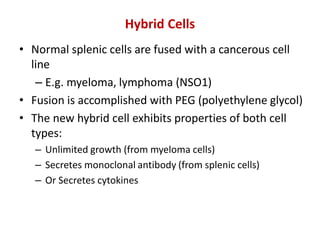
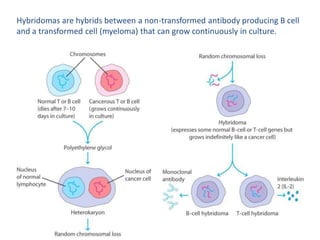
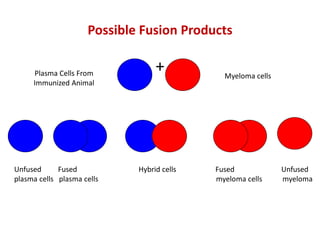
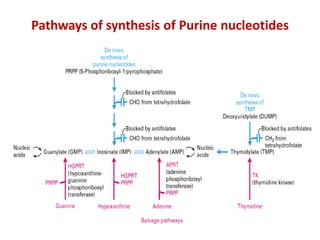
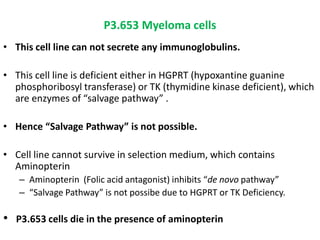
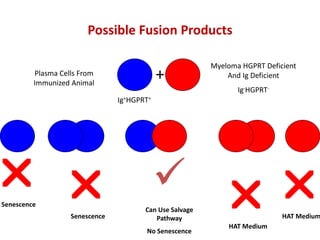
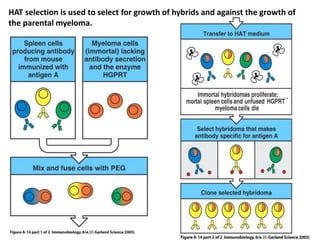
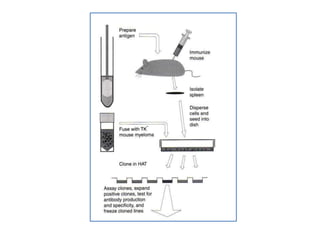
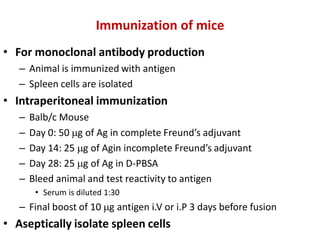
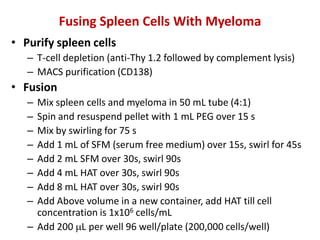
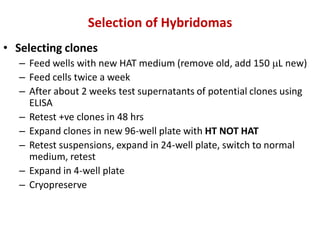
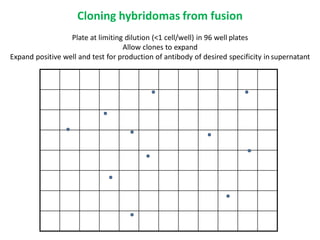
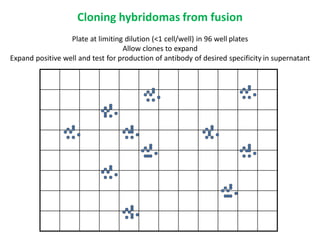
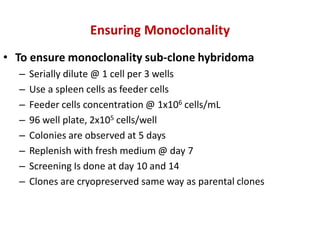
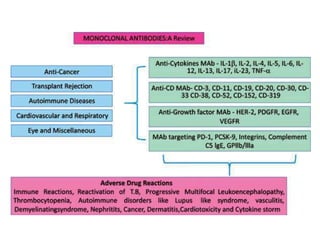
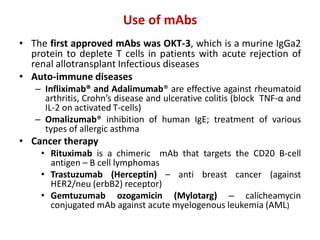
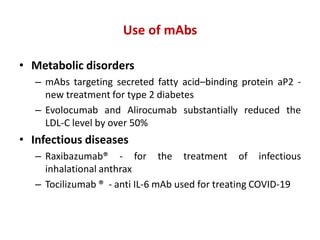
Hybridoma technology allows the production of monoclonal antibodies by fusing antibody-producing B cells with myeloma cells. This results in immortalized hybridoma cell lines that continuously secrete antibodies of a single specificity. Monoclonal antibodies are produced by immunizing mice and fusing their spleen cells with myeloma cell lines deficient in enzymes required for nucleotide synthesis. The hybridomas are selected using HAT medium, which allows for their survival and expansion. Monoclonal antibodies have various applications including treatment of cancer, autoimmune diseases, and infectious diseases.