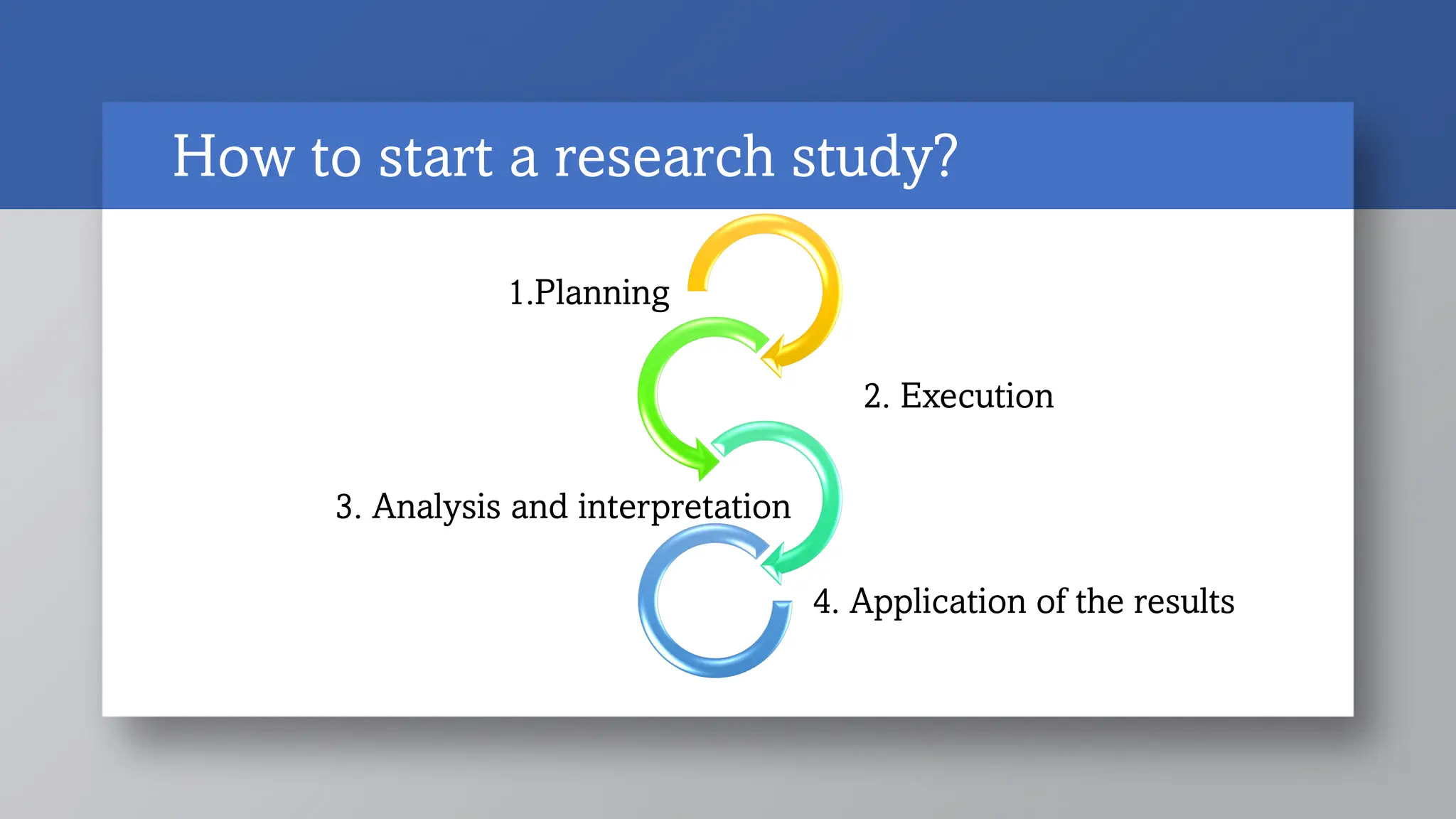
This document outlines the basic steps for planning a research study:
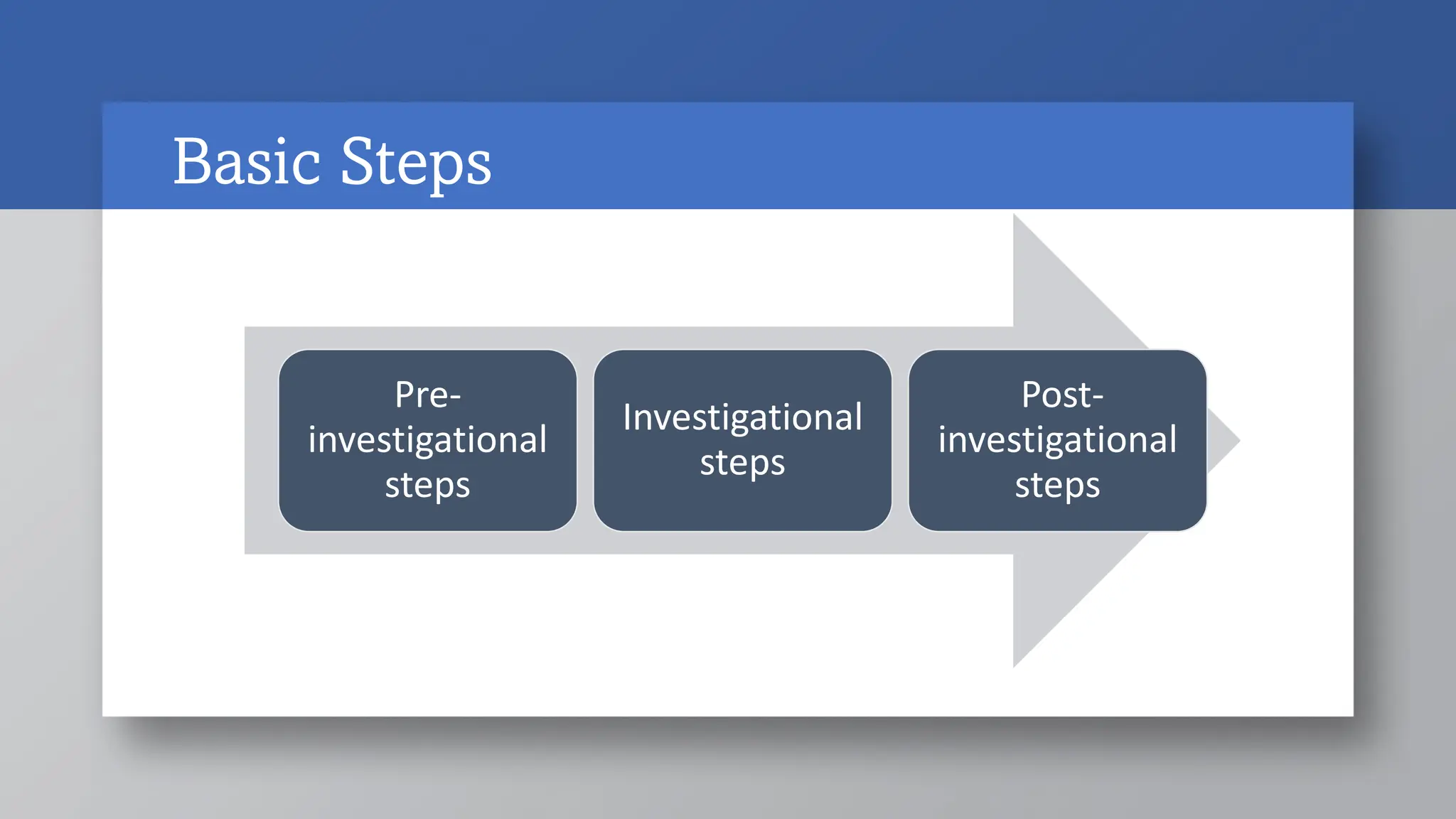
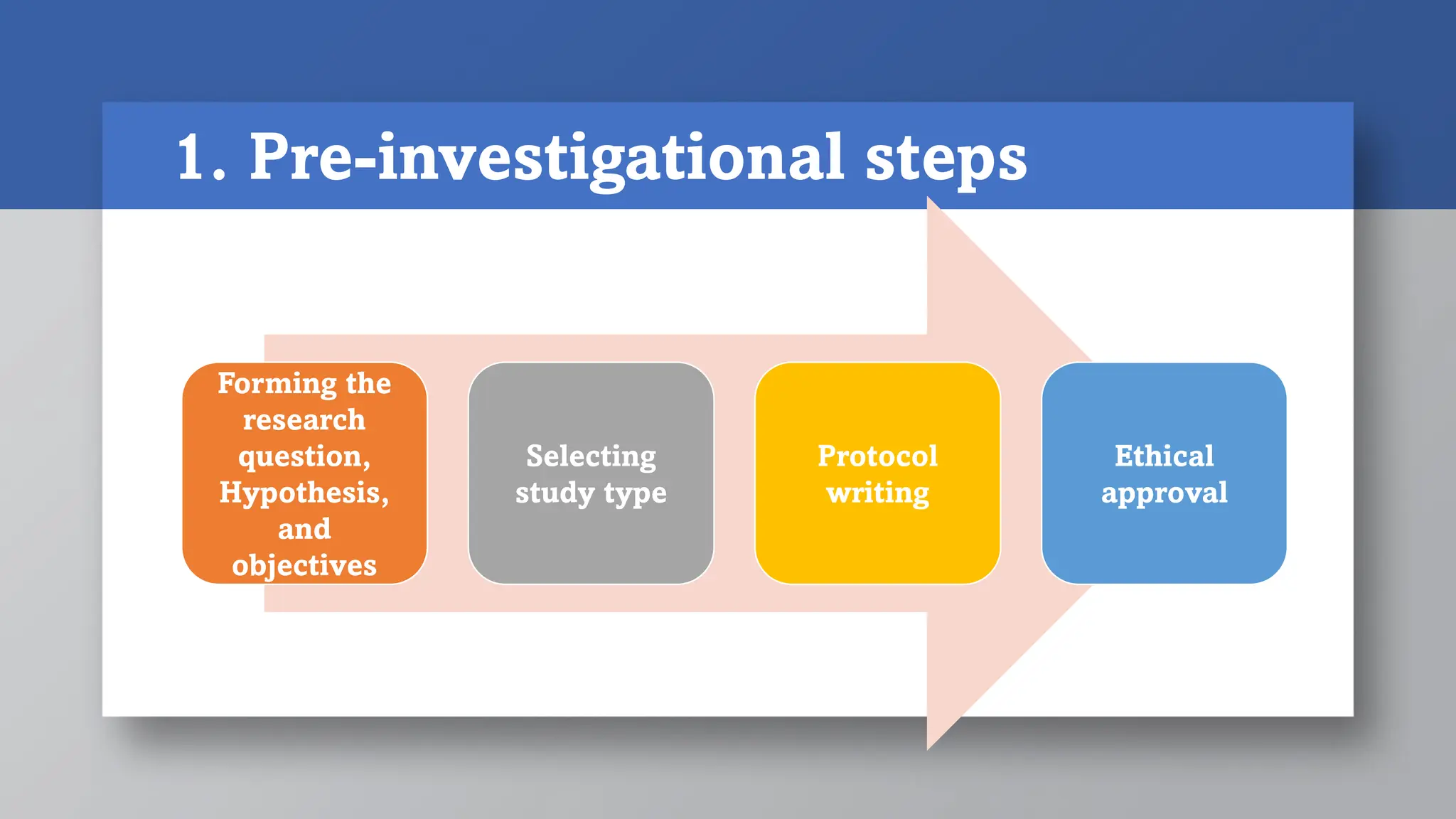
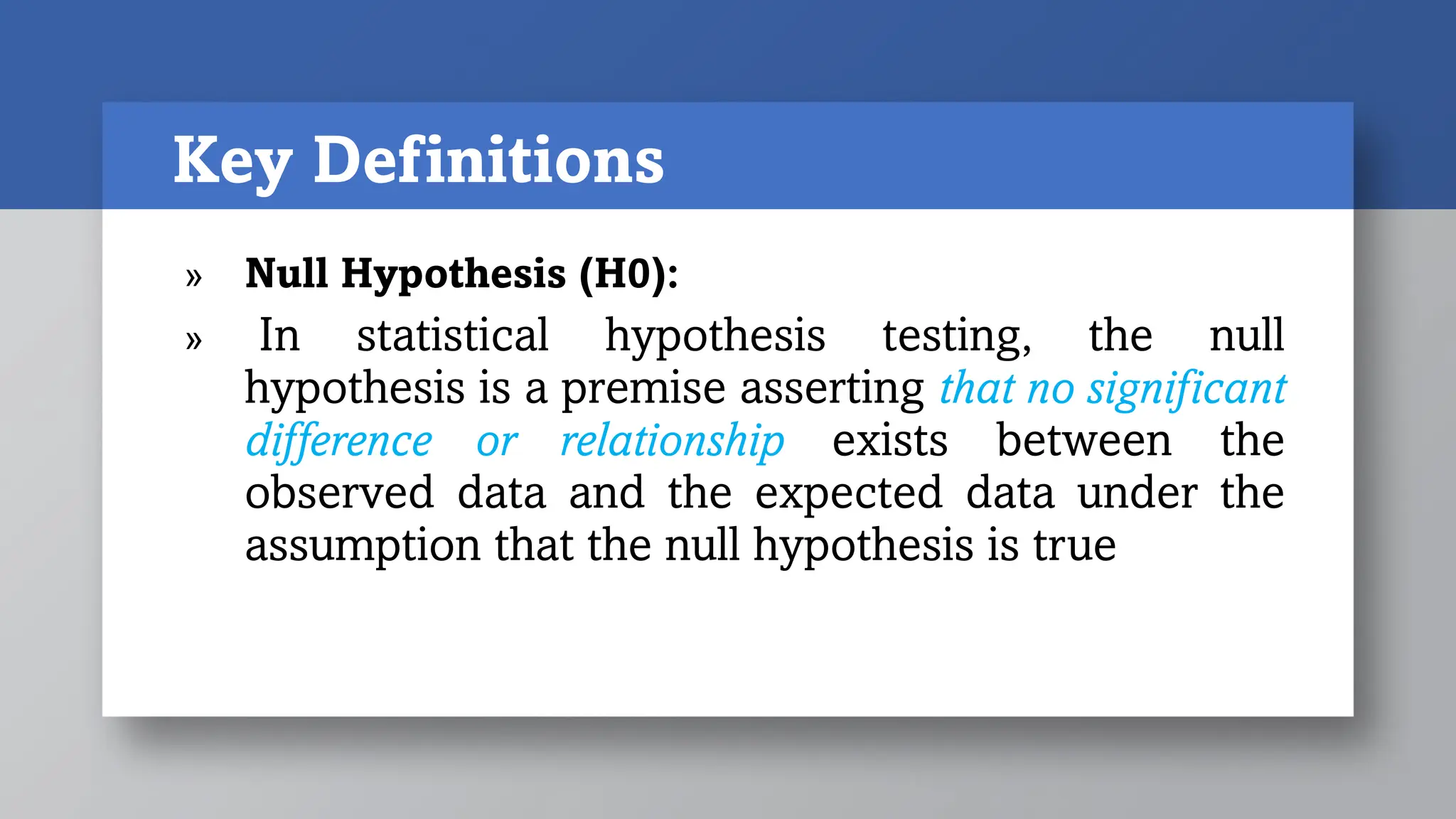
1) Pre-investigational steps include forming the research question, hypothesis and objectives, selecting a study type, and writing the study protocol. Key aspects are ensuring the research question is feasible, interesting, novel, ethical and relevant.
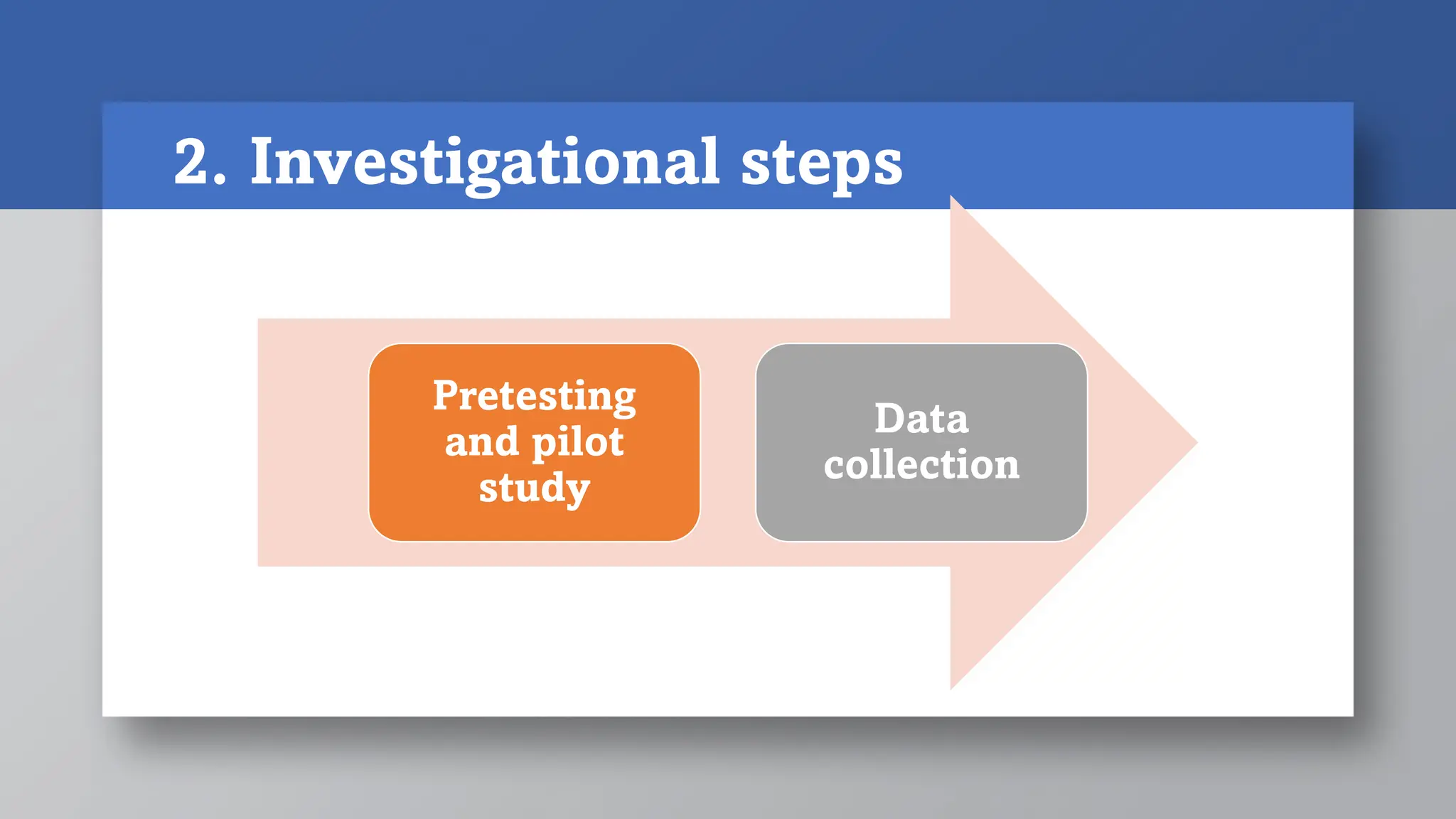
2) Investigational steps involve pretesting/piloting the study and collecting data. Pilot studies test the methodology.
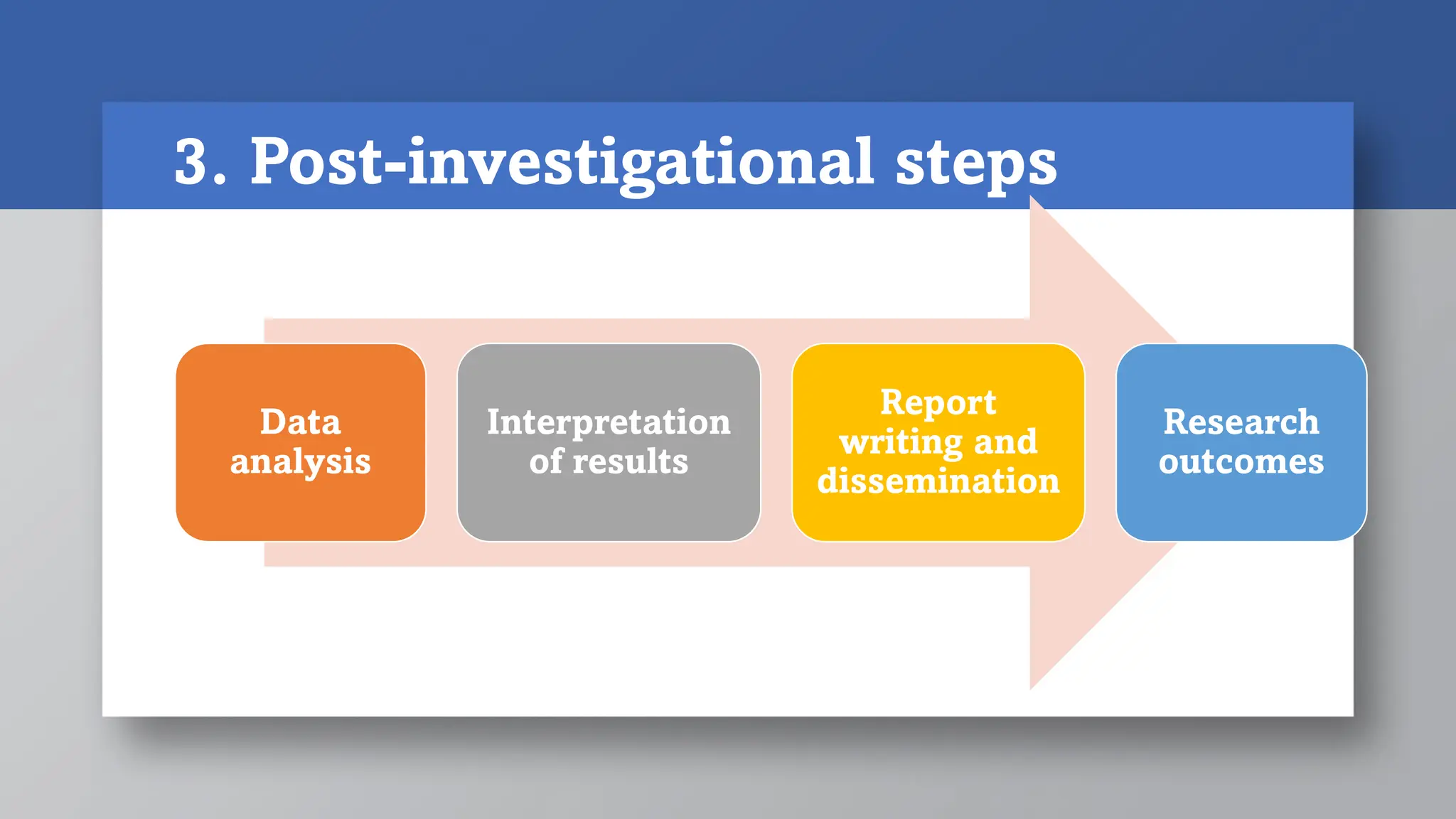
3) Post-investigational steps are analyzing and interpreting the data, writing a report, and disseminating results and research outcomes. The overall process provides a methodical framework and roadmap for conducting research.