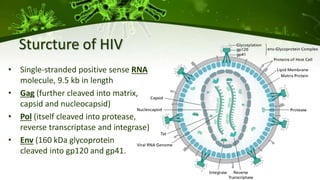
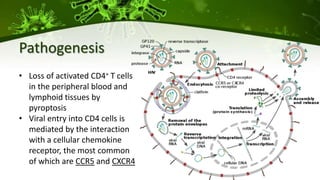
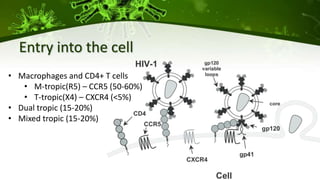
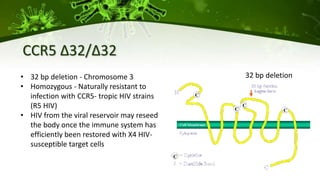
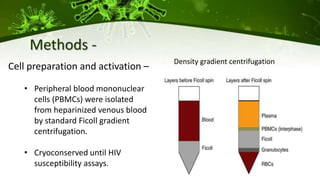
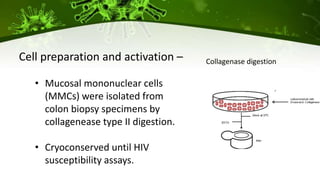
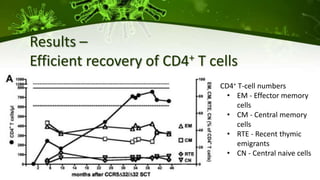
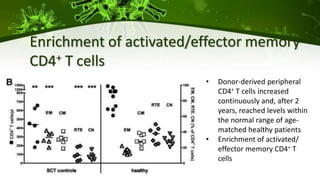
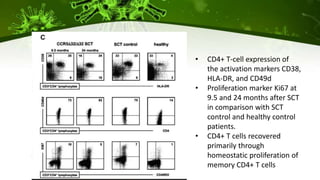
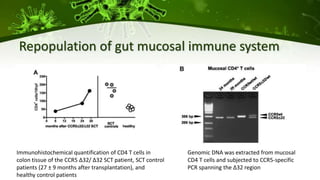
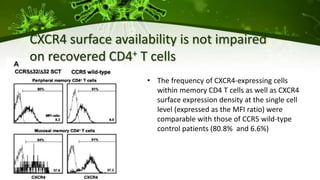
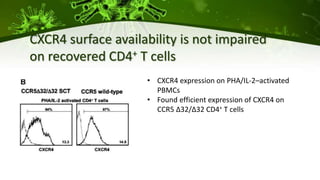
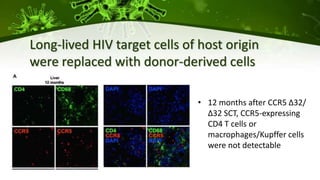
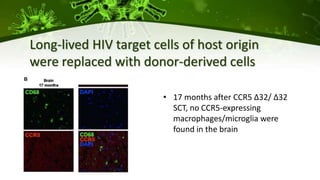
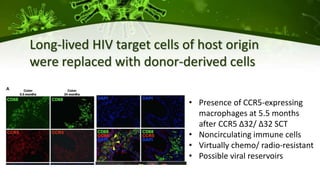
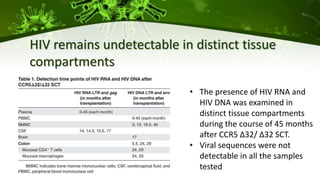
The document discusses a case study of a 40-year-old man with HIV infection and acute myeloid leukemia who underwent stem cell transplantation using donor cells homozygous for the CCR5 ∆32/∆32 mutation, which provides resistance to CCR5-tropic HIV strains. Post-transplantation, the patient showed reconstitution of CD4+ T cells without detectable HIV RNA or DNA, demonstrating a potential curative approach through this method. The findings emphasize immune system recovery's importance for sustaining the absence of HIV post-treatment and the persistence of CD4+ T cells being susceptible to x4 HIV strains.