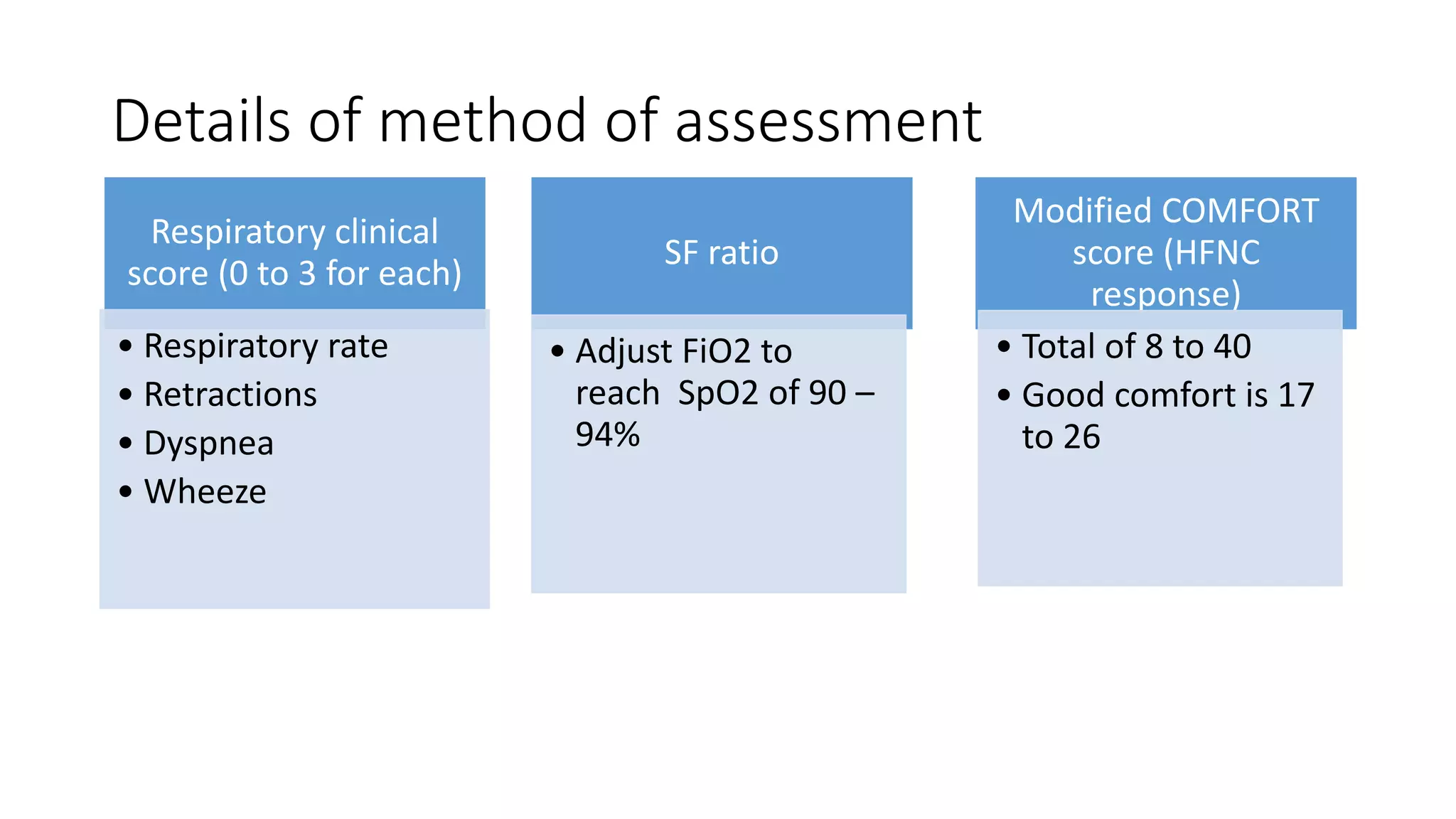
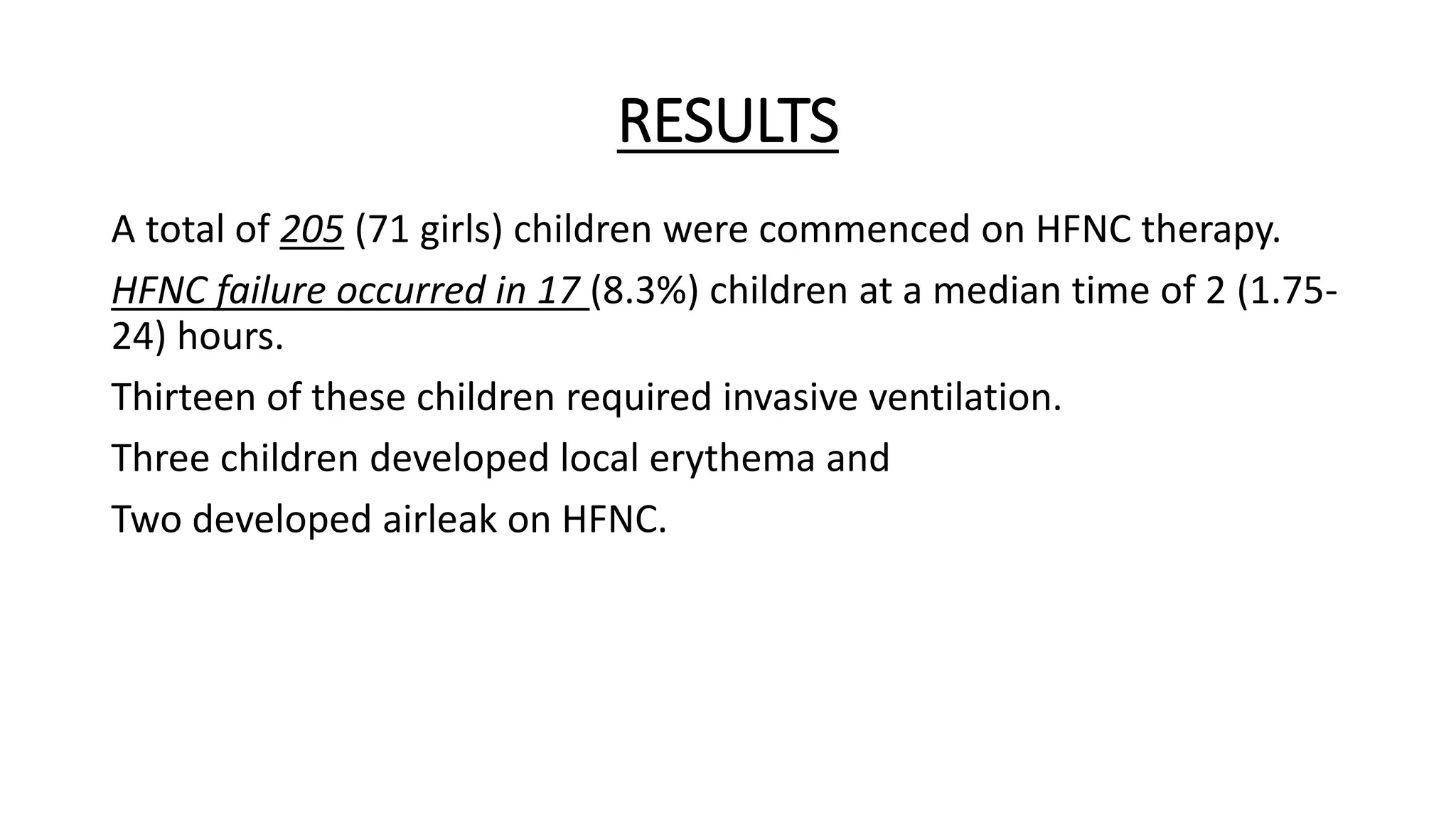
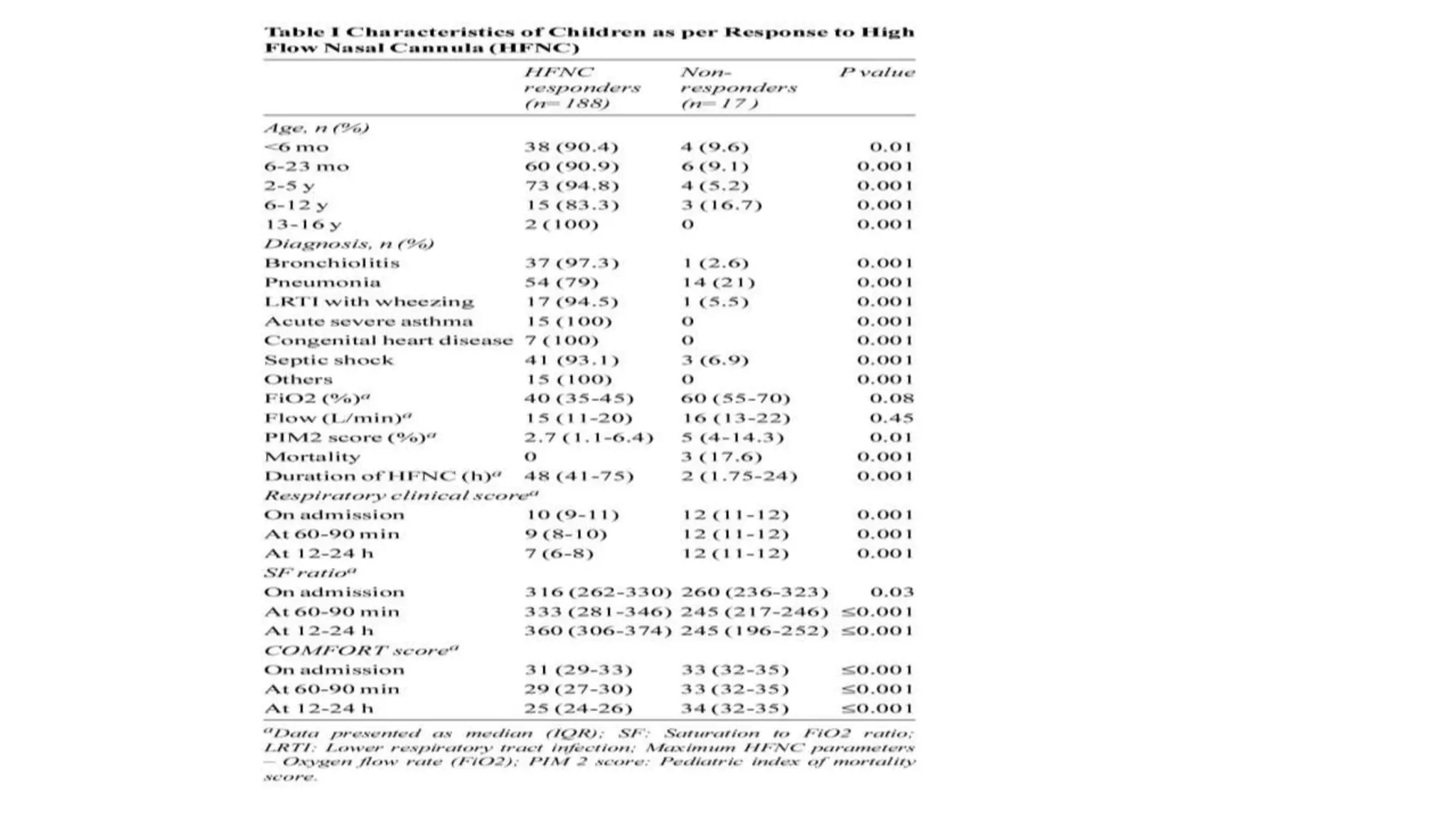
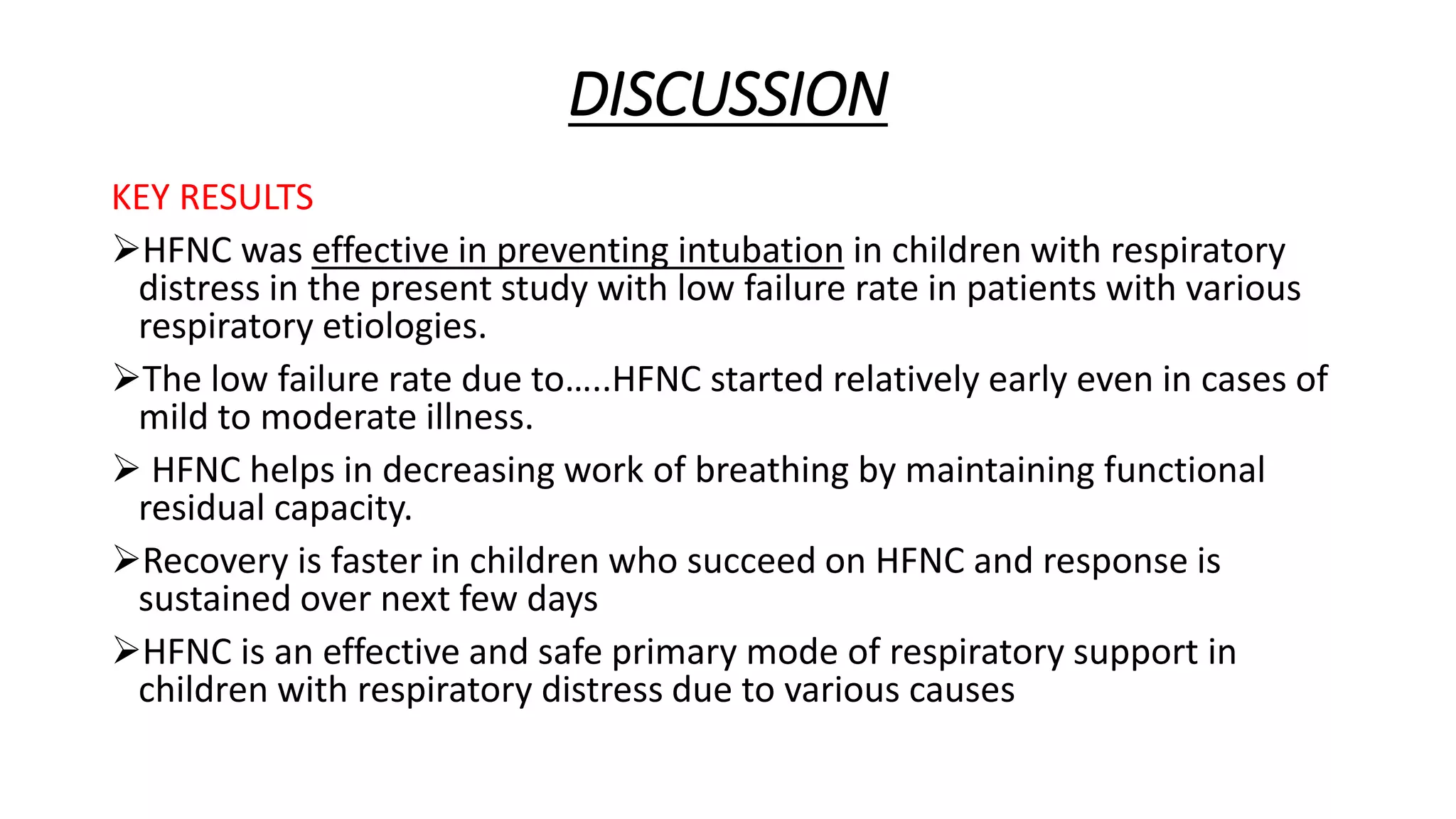
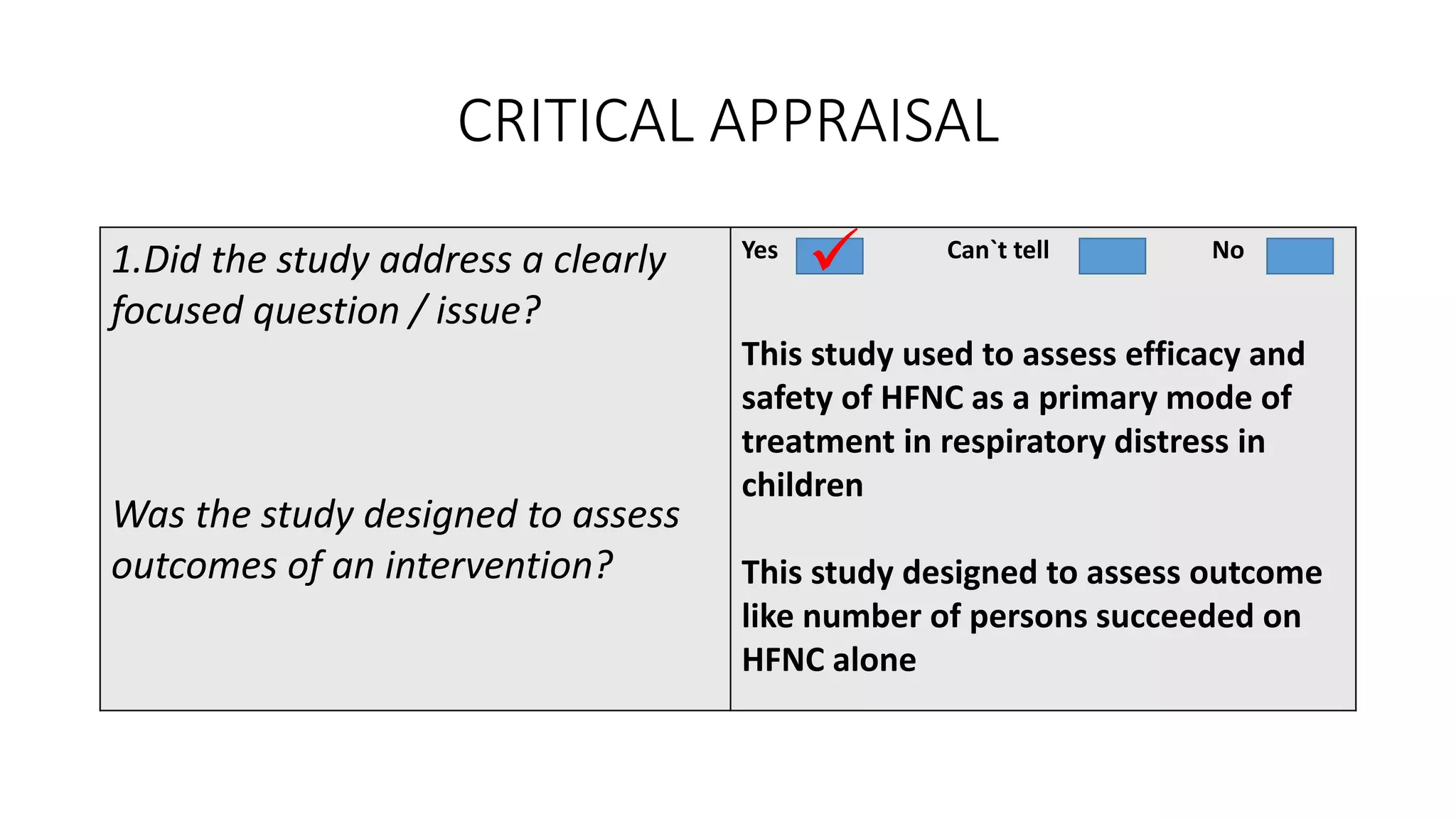
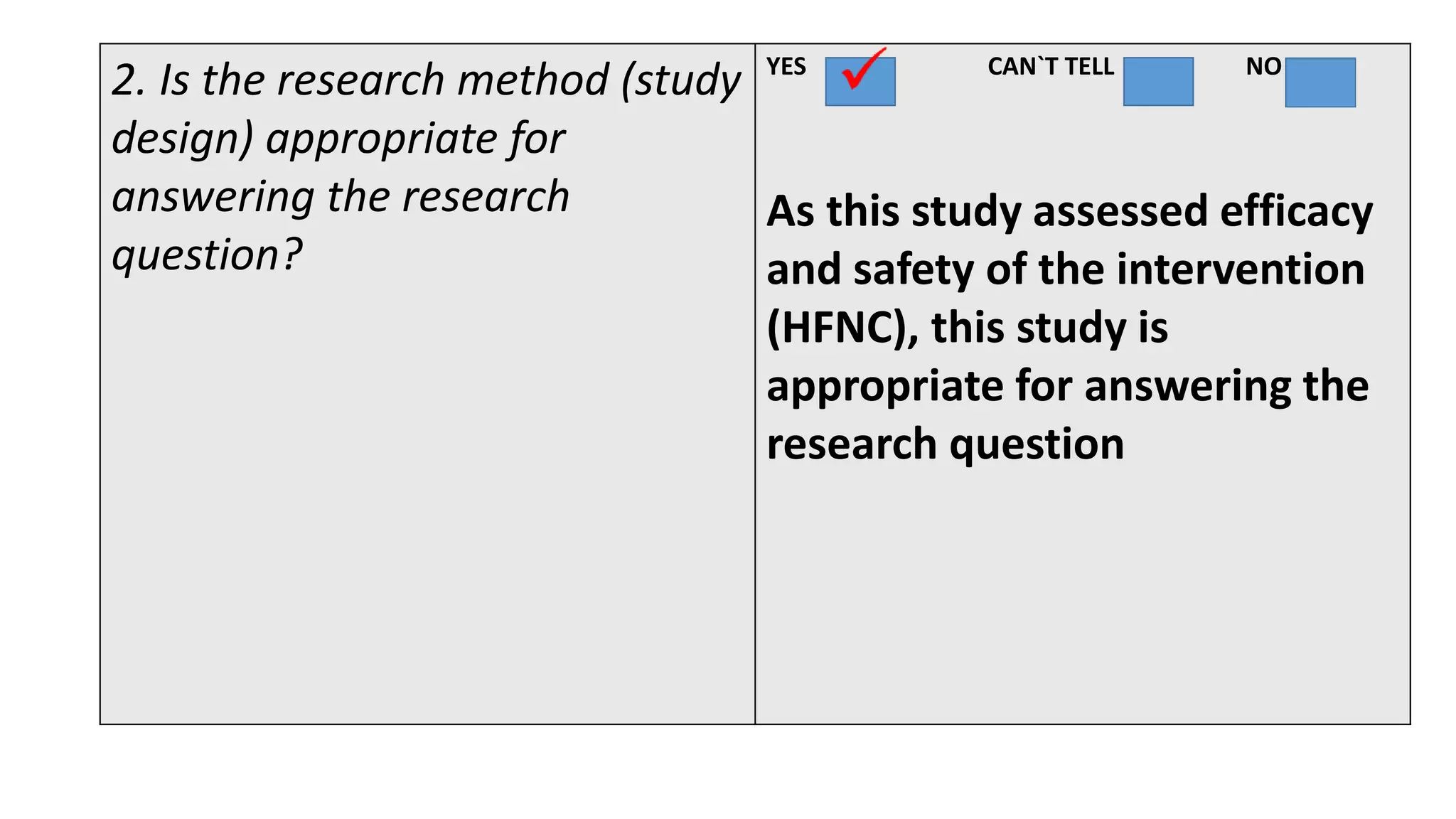
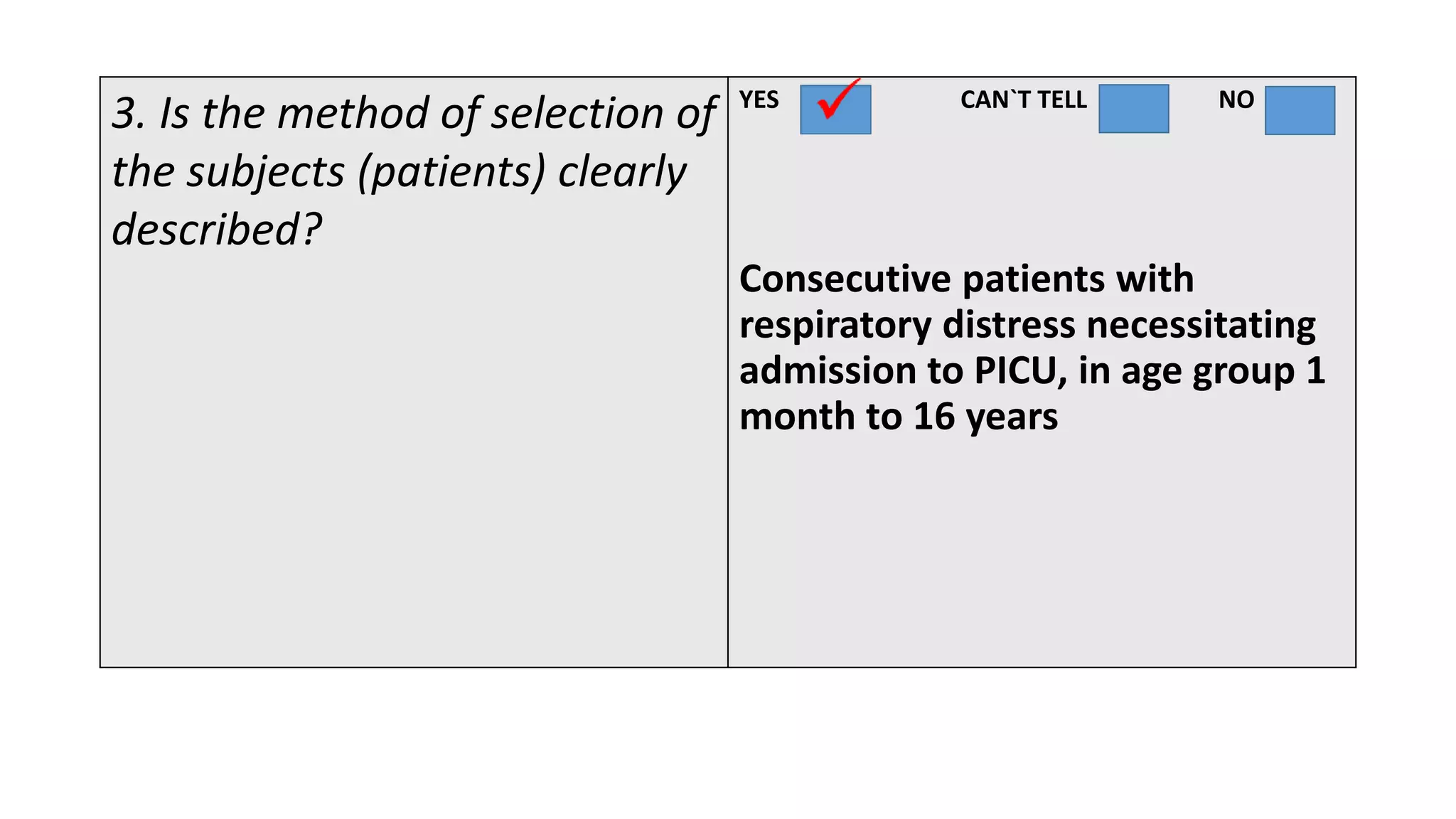
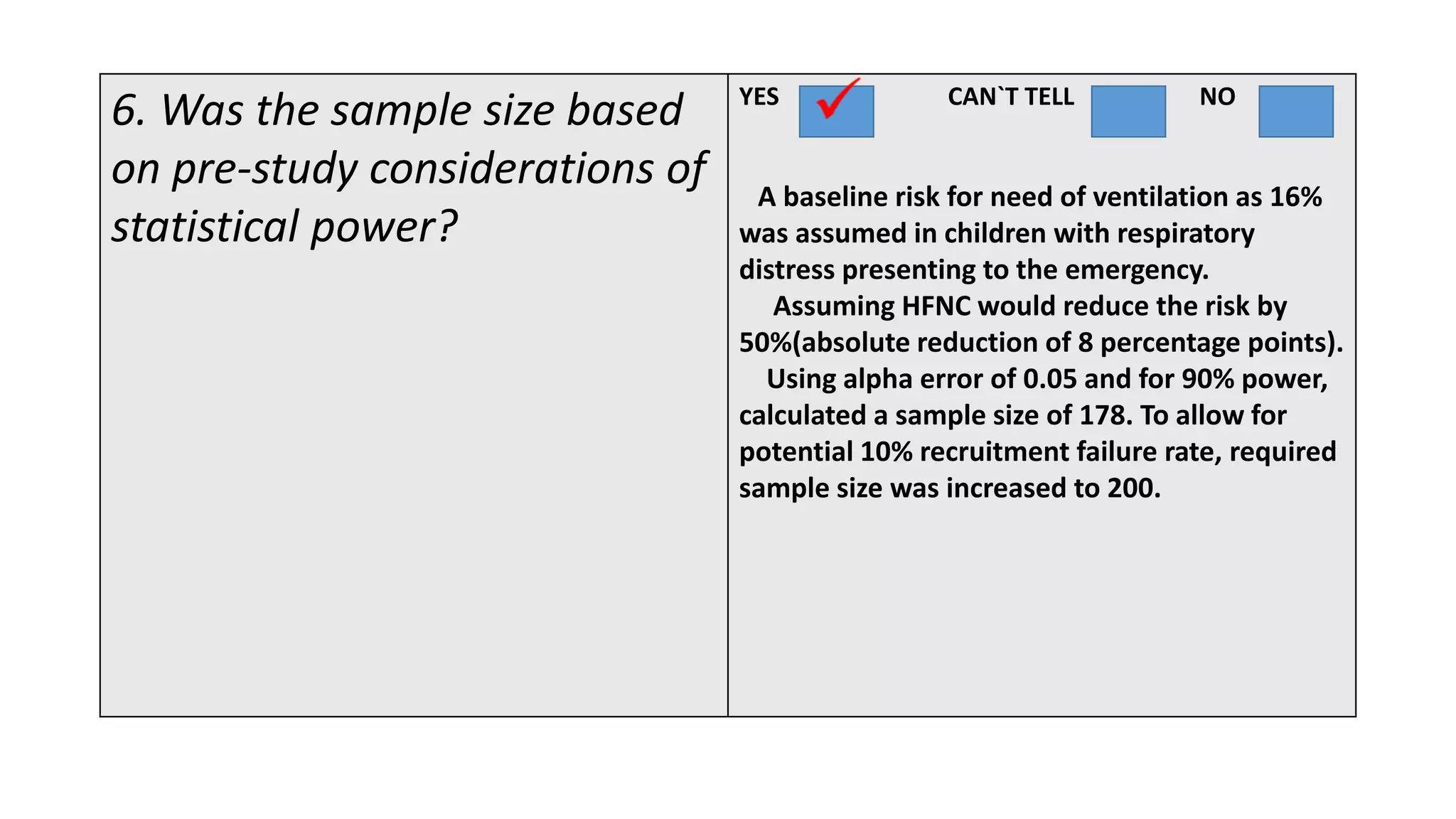
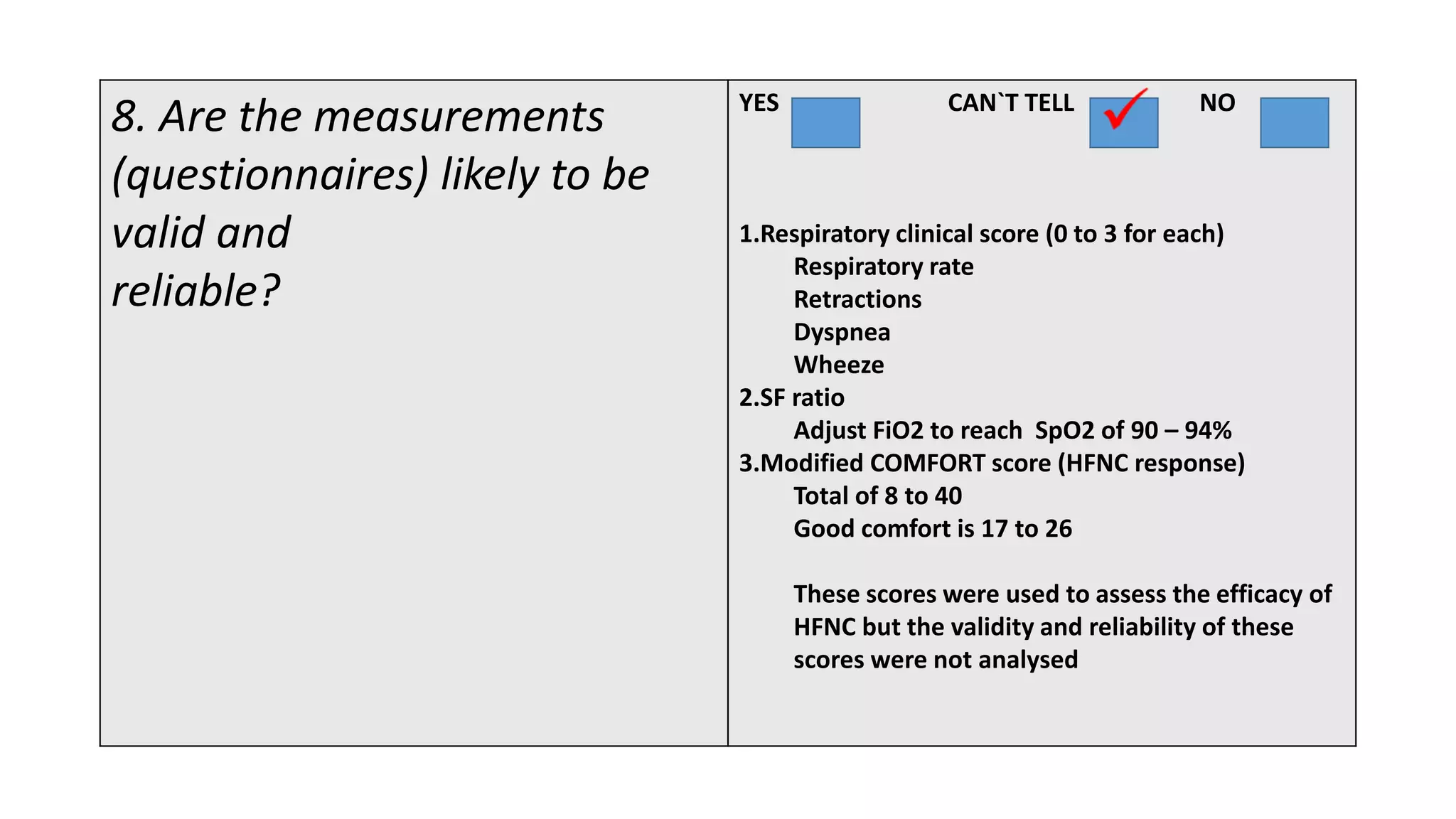
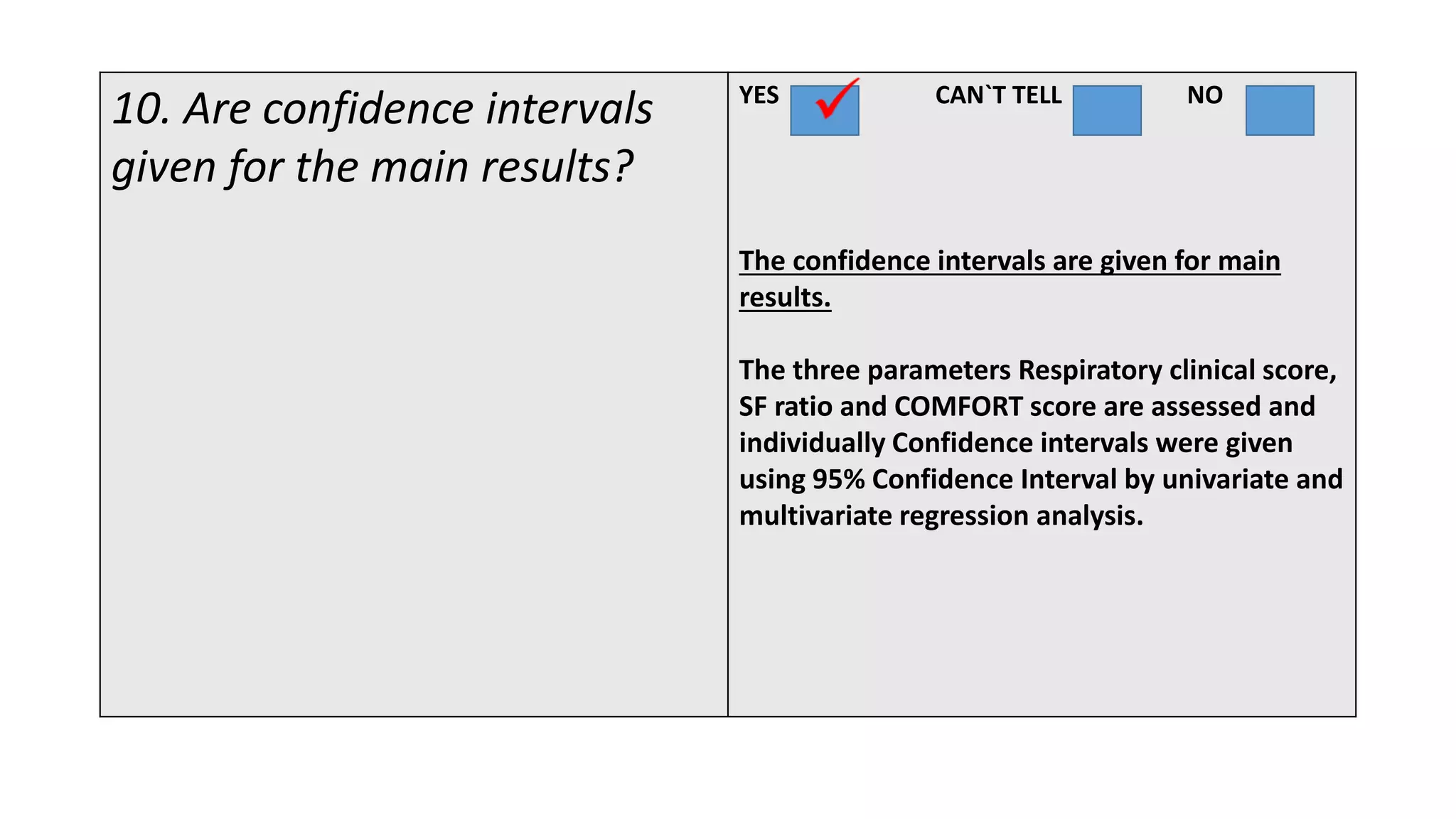
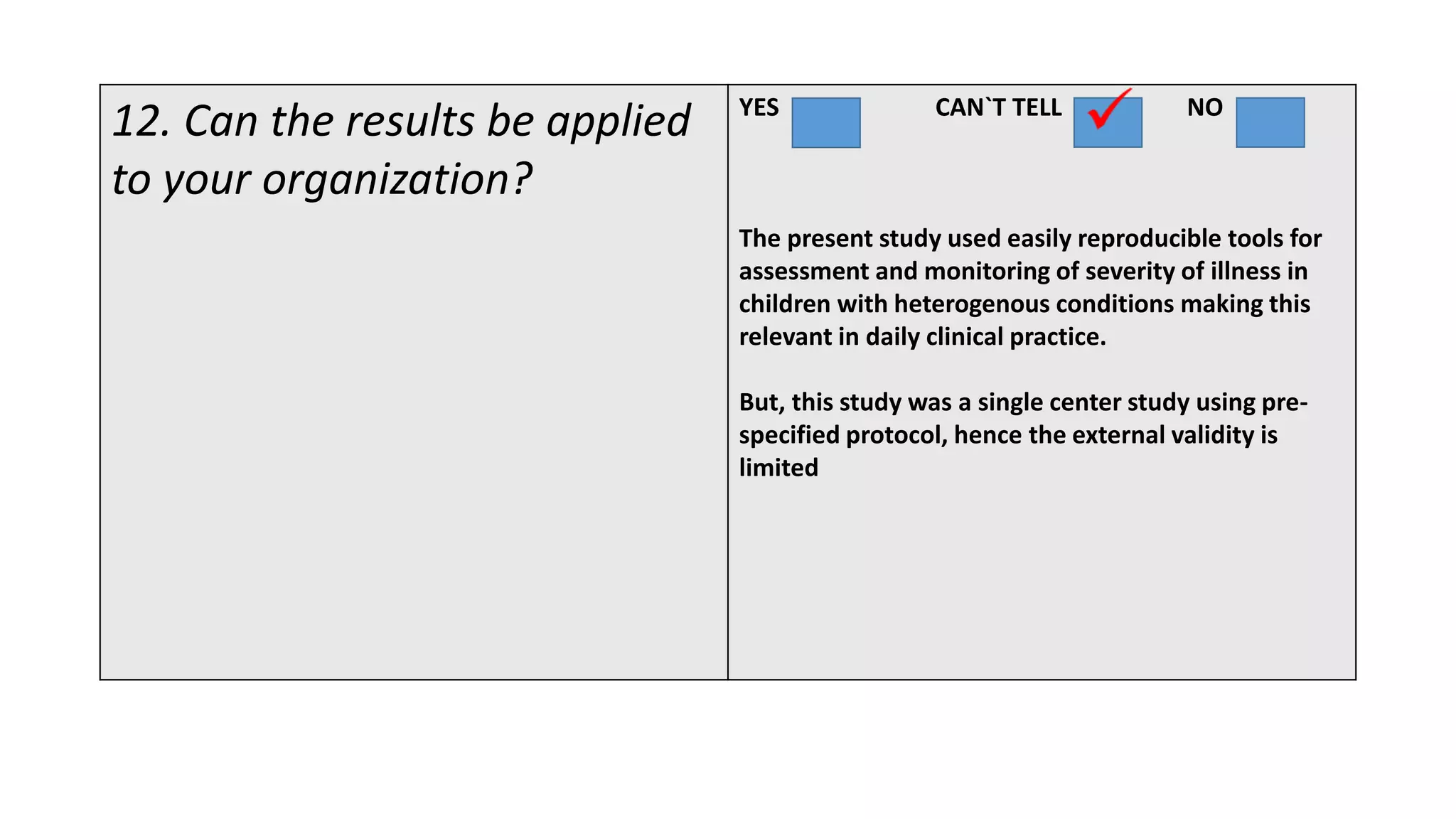
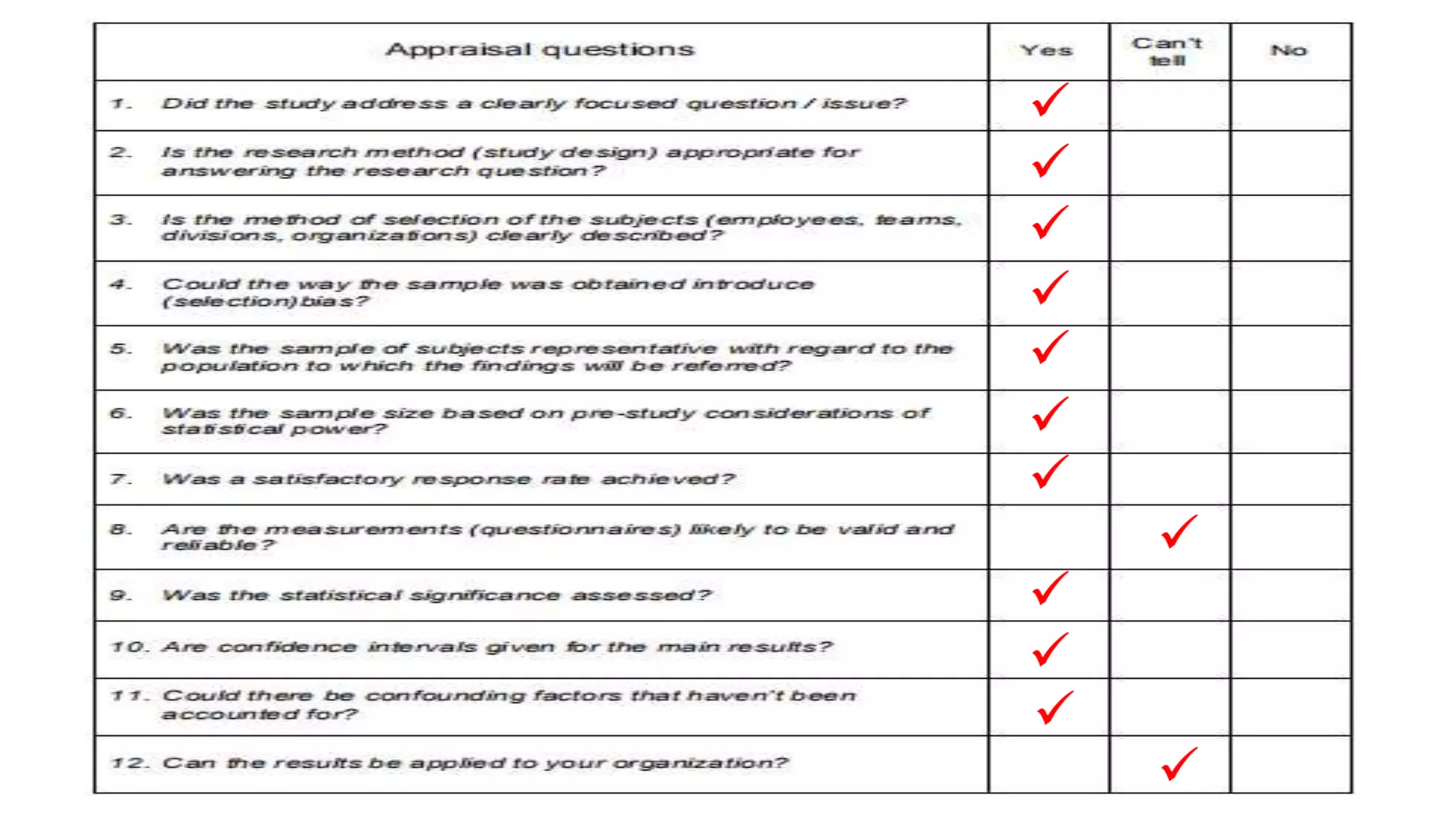
This study assessed the efficacy and safety of high flow nasal cannula (HFNC) therapy as primary respiratory support in children with respiratory distress. 205 children were given HFNC therapy, with failure occurring in 17 children requiring invasive or non-invasive ventilation. HFNC was found to be an effective and safe primary mode of respiratory support, with a low failure rate of 8.3% and most children showing a favorable response within the first few hours. However, as a single center study, the external validity is limited.