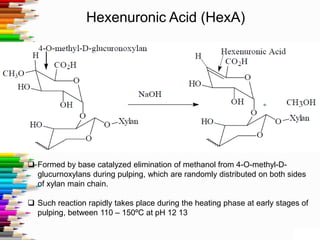
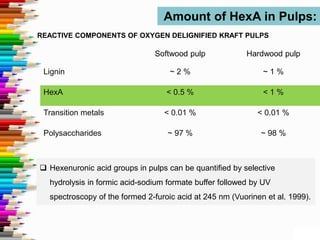
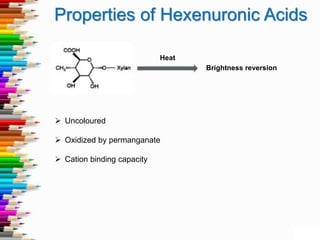
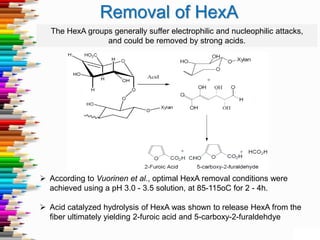
This document discusses hexenuronic acid (HexA) in pulping and bleaching. HexA is formed during pulping and increases bleaching chemical consumption. Removing HexA through acid hydrolysis prior to bleaching can reduce costs by 50% and allow for higher brightness. However, acid treatment may reduce pulp yield by 1-2%, which is a concern.