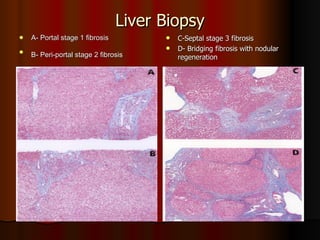
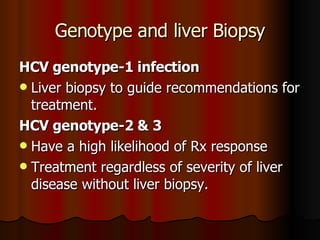
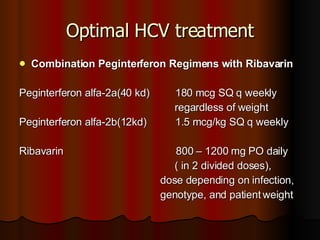
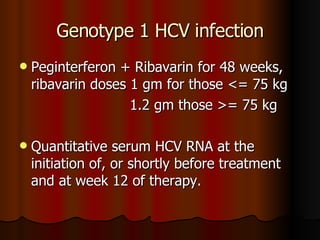
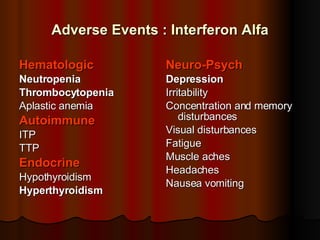
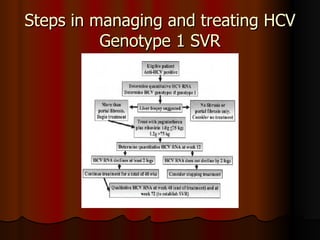
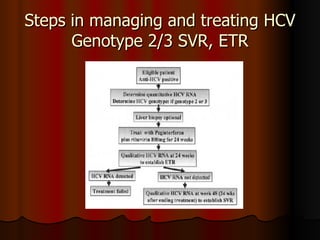
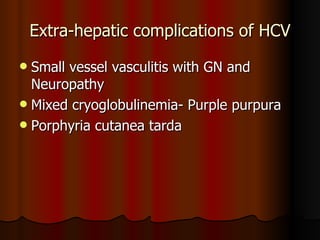
The patient has abnormal liver enzymes and tested positive for hepatitis C virus antibodies and RNA. She has a history of injection drug use 18 years ago. Her current rash and abnormal liver enzymes suggest she may have cryoglobulinemia associated with her hepatitis C infection. Measuring her serum cryoglobulins would be the most appropriate next step to evaluate for this potential extrahepatic manifestation of hepatitis C.