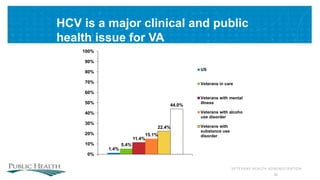
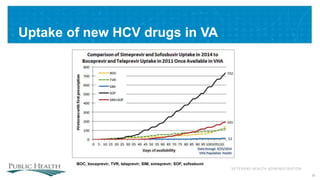
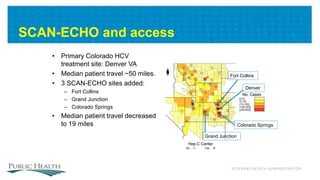
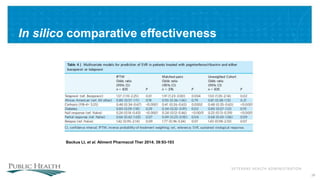
The document outlines a web conference focused on the effectiveness and value of new hepatitis C drugs, convened by the Center for Medical Technology Policy and the Institute for Clinical and Economic Review. Key discussions included the need for additional evidence to inform decisions by patients, clinicians, and payers, as well as the importance of real-world effectiveness and safety data in treatment approaches. Various experts presented on topics including clinical trial designs, treatment regimens, and strategies for optimizing patient outcomes.