This document summarizes key information about biologic therapies for psoriasis:
1) It lists the approved biologics for psoriasis - etanercept, adalimumab, infliximab, and ustekinumab.
2) It provides estimates for market exclusivity periods for these drugs in the EU and US based on patent information.
3) It details the licensed indications and dosing regimens for each drug for various conditions including psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
4) It compares the annual costs per patient and global sales for each drug based on ex-factory prices and wholesale acquisition costs.






































































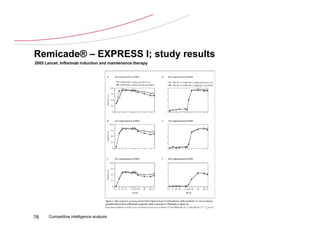
![Remicade® – EXPRESS I; study design
2005 Lancet, Infliximab induction and maintenance therapy
Double blind core study Follow-up period
scr x 2 6 10 14 22 24 26 30 38 46 50 66 weeks
Infliximab 5 mg/kg x x x x x x x x x x x
Placebo x x x x x x
PASI
PGA
NAPSI
[Infliximab
serum]
Infliximab antibodies
Anti-nuclear & anti-double
stranded DNA antibodies
71 Competitive intelligence analysis x 5 mg/kg adminsitration](https://image.slidesharecdn.com/psoriasispharmascapecv-130227013401-phpapp02/85/Psoriasis-pharmascape-cv-71-320.jpg)