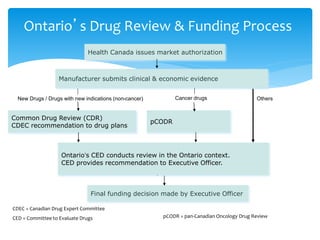
This document summarizes a presentation given by Maureen Smith on patient involvement in drug coverage reviews in Ontario. It outlines how patient groups can submit evidence to be considered by the Committee to Evaluate Drugs, including registering as a patient group, using the submission template, and meeting submission deadlines. It also provides suggestions for making submissions more impactful, such as prioritizing the most important impacts of a disease and treatment outcomes. The goal is to systematically incorporate the patient perspective into drug review and funding decisions in Ontario.