Helicobacter pylori infection is associated with increased risk of gastric cancer development. A prospective study of 1526 Japanese patients found:
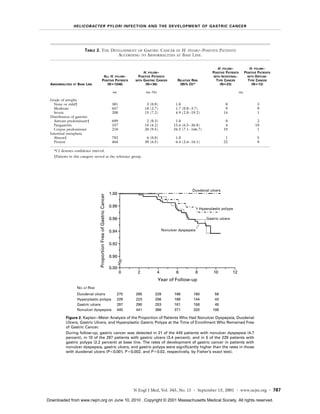
1) Gastric cancer developed in 36 (2.9%) of infected patients but none of 280 uninfected patients over 7.8 years of follow up.
2) Infected patients with severe gastric atrophy, corpus-predominant gastritis, or intestinal metaplasia were at highest risk.
3) Gastric cancer risk was highest in infected patients with nonulcer dyspepsia (4.7%), gastric ulcers (3.4%), or gastric polyps (2.2%) but none in those with duodenal ulcers.