This document contains a table of contents for a chemistry textbook that covers 8 topics related to chemistry. The table of contents lists the learning outcomes, introduction and activities for Topic 1 which is about the composition of air and the importance of oxygen. It describes the main gases that make up air, including nitrogen, oxygen and carbon dioxide. Experiments are presented to test the solubility of oxygen and carbon dioxide in water and their reactions with sodium hydroxide. The importance of oxygen for respiration is also discussed.







































![TOPIC 2 METALS W 3
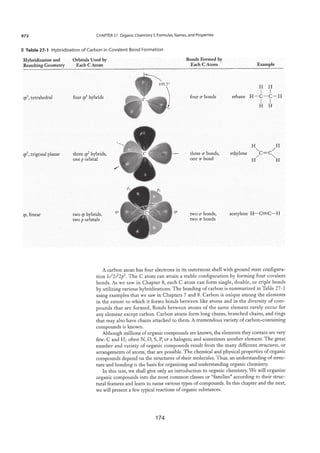
2.1.1 Structures of Metals and Alloys
Pure metals have the following properties;
x They usually have high melting and boiling points. This is due to the strong
attraction between the positive metal ions and the mobile clouds of electrons.
x They conduct electricity due to the mobile electrons (electrons cloud) within
the metal structure. When a metal is connected in a circuit, the electrons move
towards the positive terminal.
x They are malleable and ductile. If a force is applied to a metal, rows of ions
can slide over one another. They reposition themselves and the strong bonds
re-form as shown in Figure 2.3.
Figure 2.3: The positions of the positive ions in a metal before and after a
force has been applied
[Source:ȱhttp://www.chemȬisȬ
try.org/materi_kimia/struktur_atom_dan_ikatan/jenis_struktur_atom/s
truktur_logam/]
x They have high densities, as the atoms are arranged in order and closely
packed together as can be seen in Figure 2.4.
Figure 2.4: Arrangement of ions in a metal
[Source: http://martinmm.wiki.manheimcentral.org/84]
43](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-42-320.jpg)
![X TOPIC 2 METALS
4
Different metals show different types of packing and in doing so they produce the
arrangement of atoms shown in Figure 2.5.
ȱȱȱȱȱ
ȱ
ȱȱȱȱȱȱ
ȱ
ȱ
Figure 2.5: Relating different structures to the density of metal
[Source:
http://www.substech.com/dokuwiki/doku.php?id=metals_crystal_structure]
Alloys are a mixture of;
x Two or more metals (for example, brass is an alloy of zinc and copper); or
x A metal and non-metal (for example, steel is an alloy of iron and carbon).
Figure 2.6 shows the alloy structure. The blue circles represent atoms of metal A and
the white circles are atoms of metal B which is added to make the alloy. These
different atoms give the alloy different physical properties from that of the pure metal.
ȱȱȱȱȱȱȱȱȱȱ
Figureȱ2.6:ȱStructureȱofȱanȱalloyȱ
[Source:ȱhttp://www.chem.qmul.ac.uk/surfaces/scc/scat6_4.htm]ȱ
ȱ
Atom of metal A
Atom of metal B
44](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-43-320.jpg)
![TOPIC 2 METALS W 5
Alloys are formed by mixing the molten substances thoroughly. But why make alloys?
The reasons why alloys are made are:
(a) To increase the strength and hardness of a pure metal. The presence of the
2.2
atoms of other elements disrupts the orderly arrangement of the pure metal.
The layers of metal atoms are prevented from sliding over one another easily.
This makes alloys stronger and harder than pure metals.
(b) To increase the resistance to corrosion of a pure metal. Alloying can prevent
metals from corrosion. This is because alloying helps to prevent the formation
of oxide layer on the surface of the metal (We will discuss the reaction of
metals in subtopic 2.2).ȱ
(c) To improve the appearance of a pure metal. Alloying helps to keep the metal
maintain the glossy nature of the surface as it prevents the formation of the
metal oxide.
Table 2.1 shows some of the more common alloys with their composition.
ȱ
Table 2.1: Composition of common alloys
[Source: Ryan (2001)]
Alloy Composition
Brass 65% copper, 35% zinc
Bronze 90% copper, 10% tin
Cupro-nickel 30% copper, 70% nickel
Duralumin 95% aluminium, 4% copper,1% magnesium, manganese and
iron
Magnalium 70% aluminium, 30% magnesium
Pewter 30% lead, 70% tin, a small amount of antimony
Solder 70% lead, 30% tin
CHEMICAL PROPERTIES OF METALS
The metals in ores are chemically bonded to other elements. So how can we extract
the metals? To answer this, we must understand the Reactivity Series of metals. In
the Reactivity Series, the most reactive metals are at the top. The less reactive ones are
at the bottom. We can start putting the metals in order by looking at their actions with
heat, water and dilute hydrochloric acid.
45](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-44-320.jpg)
![X TOPIC 2 METALS
6
2.2.1 Chemical Reaction of Metals with Heat
Conduct Experiments 2.1 and 2.2 to judge the reactivity by putting the metals into
competition with each other. In these two experiments, the metals will “fight” each
other to “win their prize” which is oxygen. The more reactive metal will win the
fight.
ȱ
Experiment 2.1
1. Mix a spatula of iron fillings and copper oxide
in a test tube. Heat the mixture strongly
x Is there a reaction? Look for a red glow
spreading through the mixture.
2. When the tube has cooled, empty it into a dish.
x Can you see any brown copper metal left?
[Source: Ryan (2001)]
Copper starts off with the oxygen in copper oxide. However, iron is more reactive, so
it takes the oxygen away from copper. We say that iron has displaced (“kicked out”)
the copper.
Copper oxide + iron Æ iron oxide + copper
CuO(s) + Fe(s) Æ CuO(s) + Cu(s)
This is a displacement reaction. It shows us that iron is more reactive than copper.
ȱ
SELF CHECK 2.2
In Experiment 2.1, what do you expect will happen if we change:
x copper oxide with iron; and
x iron with copper?
Will there be any reaction? Why?
There actually will not be a reaction between iron oxide and copper because copper is
less reactive than iron.
46](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-45-320.jpg)
![TOPIC 2 METALS W 7
You can now try some other displacement reactions as in Experiment 2.2.
ȱȱ
ȱ
ȱȱ
ȱ
ȱȱȱȱ
Experiment 2.2
x Try heating the mixtures of metals and oxide
shown in the table:
x Look for any signs of reaction. Tick (—) in the
“Reaction Table” if there is a reaction.
(Be careful when looking for signs of reaction.
Zinc oxide turns yellow when you heat it by
itself. It turns white again when it cools down).
x Write word equations for the reactions you
have ticked)
Metal/
Metal
oxide
Zinc
oxide
Zinc
Iron
Copper
Magnesium
Reaction Table
2.2.2 Chemical Reaction of Metals with Water
Iron
oxide
Copper
oxide
You have already seen how the action of heat with metals in the displacement
reaction. Now, you can arrange the order of the reactivity of metals iron, zinc, copper
and magnesium:
i. Magnesium
ii. Zinc
iii. Iron
iv. Copper
We can also judge reactivity by observing the metal’s reaction with water. Let us look
at the reaction of lithium, sodium and potassium with water.
Experiment 2.3
1. Put water in three different glass basins.
2. Drop small pieces of
x Lithium in basin 1
x Sodium in basin 2
x Potassium in basin 3
3. Collect the gas given off as shown;
x Test the gas with a lighted splint
4. Test the solution formed with red litmus
paper.
x Is the solution left acidic or alkaline?
[Source: Ryan (2001)]
From Experiment 2.3, you can observe that lithium moves slowly on the surface of the
water, while sodium melts to become a small sphere, move rapidly and randomly on
47](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-46-320.jpg)
![X TOPIC 2 METALS
8
the water surface with a hissing sound as it reacts. Potassium gets so hot that it lights
the hydrogen gas that water gives off. It burns with a lilac flame, move very rapidly
and randomly on the water surface with a hissing and popping sound. The colourless
solution formed turns red litmus paper to blue.
When red litmus paper turns to blue,
the solution formed is an alkaline!
The chemical equation for the reaction of lithium with water is as follows:
Lithium + Water Æ Lithium hydroxide + Hydrogen
2Li(s) + 2H2O (l) Æ 2LiOH (aq) + H2 (g)
SELF-CHECK 2.3
Write the word and symbol equations for sodium and potassium
reacting to water.
In the case of magnesium, this metal normally reacts slowly with water. But we
can speed up the reaction by heating up the water to make steam as in Experiment
2.4.
Experiment 2.4
1. Heat the magnesium strongly.
Every now and again, switch the
flame briefly to the ceramic wool
to make a steam.
2. As the reaction starts, the gas
given off can be lit at the end of
the tube.
x Can you name the gas?
[Source: Ryan (2001)]
The magnesium reacts strongly with the steam. It leaves white magnesium oxide in
the test tube. Hydrogen gas is given off.
48](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-47-320.jpg)
![TOPIC 2 METALS W 9
Magnesium + Steam Æ Magnesium oxide + Hydrogen
Mg (s) + H2O (g) Æ MgO(s) + H2 (g)
The oxygen atom in H2O has “swapped partner”! It start off with hydrogen, but ends
up with magnesium.
Table 2.2 gives the different observations when metals react with water and steam.
Table 2.2: Reaction of metals with water and steam
T
a
b
l
e
2
.
2
Metals Reaction with Water Reaction with Steam
Potassium
Sodium
Lithium
Calcium
Fizz, giving off hydrogen gas and
leaving an alkaline (hydroxide)
solution.
Explode
Magnesium
Aluminium
Zinc
Iron
Very slow reaction.
(Aluminium is protected by a layer
of aluminium oxide on its surface).
React, giving off hydrogen gas and
forming the metal oxide.
2.2.3 Chemical Reaction of Metals with Diluted
Hydrochloric Acid
Another simple way to judge the reactivity of metals is to compare the reaction with
diluted acid. Metals will react quicker with diluted acid compared to water especially
the metals below calcium in Table 2.2.
Conduct Experiment 2.5 to compare the reactivity of metals when react with dilute
hydrochloric acid.
Experiment 2.5
1. Clean the metals with sand-paper.
2. Set up the boiling tube as shown:
x Can you see bubbles?
(If you see no bubbles, you can warm the
tube gently in a beaker of hot water)
3. Record your results in a table.
(Do your results agree with the order in
Table 2.3 ?)
[Source: Ryan (2001)]
Notice that copper does not react with hydrochloric acid. However, the other metals
tested do react. For example, magnesium:
49](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-48-320.jpg)


![X TOPIC 2 METALS
12
2.3
EXTRACTION OF IRON AND ALUMINIUM
ȱ
In the earlier sub-topic, we learned about the Reactivity Series. We will now look at
how to get metals from their ores. This includes iron, which is the most widely used of
all metals. Figures 2.8 and 2.9 show iron ore and the mining of iron ore.
2.3.1 Extraction of Iron
Figure 2.8: Iron ore, haematite
[Source: http://www.e-rocks.com/Products.aspx?action=showproduct&id=107003]
Figure 2.9: Mining of iron ore in Karnataka
[Source: http://khanija.kar.ncode.in/SitePages/EAuctionData.aspx]
52](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-51-320.jpg)

![X TOPIC 2 METALS
14
x The carbon monoxide then reacts with iron oxide to get iron.
Fe2O3(s) + 3CO(g) Æ 2 Fe(l) (s) + 3CO2 (g)
At the high temperature (up to 1900°C) in the furnace, the iron is in molten form
(liquid). So, it sinks to the bottom of the furnace. The iron then will run off into
mould. The molten slag floats to the top of the iron. The slag is tapped off, cooled and
used for making roads.
Figure 2.11: The blast furnace
[Source: http://images.yourdictionary.com/blastȬfurnace]
2.3.2 Extraction of Aluminium
Figure 2.12: Aluminium ore, bauxite
[Source: http://www.greenerȬ
industry.org.uk/pages/aluminium/aluminium_4PMsummary.htm]
54](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-53-320.jpg)
![TOPIC 2 METALS W 15
As shown in the Reactivity Series (refer Figure 2.10), the position of aluminium is
before carbon. This means aluminium is more reactive than carbon, so carbon cannot
be used to extract aluminium. So, how do we extract aluminium from its ore, bauxite,
which contains aluminium oxide, Al2O3?
Reactive metals can only be extracted from
their ores by electrolysis!
ȱ
ȱ
2.3.3 Extraction of Aluminium – Electrolysis of Aluminium
Oxide
ȱ
Figure 2.13 shows the electrolytic cell used for the extraction of aluminium.
Figure 2.13: Extraction of aluminium
[Source: http://www.meritnation.com/askȬanswer/question/explainȬtheȬ
processȬofȬextractionȬofȬaluminiun/metalsȬandȬnonȬmetals/2230314]
x Aluminiumȱ oxideȱ isȱmixedȱwithȱ cryolite,ȱNa3AlF6,ȱ toȱ lowerȱ theȱmeltingȱ
pointȱofȱaluminiumȱoxideȱ(2045°C)ȱtoȱaboutȱ900°C.ȱ
x Blocksȱofȱcarbonȱactȱasȱtheȱanodeȱwhileȱtheȱcarbonȱliningȱofȱtheȱcellȱactsȱasȱ
theȱcathode.ȱ
x Atȱ theȱ cathode,ȱ theȱ aluminiumȱ ionsȱ areȱ dischargedȱ toȱ formȱ aluminiumȱ
metal.ȱ
Al3+(l)ȱȱȱ+ȱȱȱ3eȱȱȱȱȱÆȱȱȱȱAl(l)ȱ
x Liquidȱaluminiumȱ isȱdenserȱ thanȱ theȱ electrolyteȱandȱwillȱbeȱ collectedȱatȱ
theȱbottomȱofȱtheȱcell.ȱ
x Atȱtheȱanode,ȱtheȱoxideȱionsȱareȱdischargedȱtoȱformȱoxygenȱgas.ȱ
2O2Ȭ(l)ȱȱȱÆȱȱO2(g)ȱȱȱ+ȱȱȱȱ4eȱ
55](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-54-320.jpg)

![TOPIC 2 METALS W 17
Another metal that has many useful properties is aluminium. It conducts heat and
electricity well. It has low density for a metal. It does not corrode.
Platinum is used in catalytic converters, fitted to car exhausts. It cuts down the
amount of pollution from cars.
A radioactive isotope of cobalt is used to treat patients with cancer.
Figure 2.14 shows some uses of common metals around the home.
Figure 2.14: Some uses of metals at home
[Source: Ryan (2001)]
ȱȱȱȱ
ACTIVITY 2.4
Look at the compund of your school. Name the metals and
the uses of metals at your school.
ȱ
ȱȱȱȱȱ
ȱ ȱ
57](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-56-320.jpg)









































































![[Source
ȱ
Ironȱ pow
powderȱ
youȱheat
ȱ
ȱȱȱȱȱȱȱȱȱȱȱ
ȱ
ACTIVITY 5
.2
Mix sulphur a
mixture. Put th
the mixture is
Theȱchem
ȱ
Ironȱ(s)ȱȱ
ȱ
ȱ
ȱ
ȱȱ
A
ȱ
ȱ
Figur
e:ȱhttp://halya
wderȱwillȱ beȱ
fromȱtheȱsulp
tȱtheȱmixture?
5 SPEED OF
and iron powde
he magnet nea
heated. What
The mixture is
a chemical reac
have now becom
er. Then, heat t
r the substance
will happen?
not attracted to th
ction has occured
me iron sulphide.
micalȱequation
+ȱȱSulphurȱ(s)
TOPIC
reȱ5.2:ȱMixture
angyuhao.blo
field
attractedȱ toȱ
phur.ȱBut,ȱdo
?ȱȱ
he
e after
he magnet anymo
d in which iron an
nȱforȱthisȱreac
)ȱȱȱÆȱȱIronȱsul
ȱ
ȱofȱsulphurȱand
ogspot.com/p/
dȬand.html]
theȱmagnet,ȱ
oȱyouȱthinkȱt
ctionȱis:,ȱ
lphideȱ(s)ȱ
CHEMICAL R
ȱ
dȱironȱpowder
/termȬ2ȬlabȬex
soȱweȱ canȱ s
theȱsameȱthin
re because
nd sulphur
REACTIONS
rȱ
xperimentsȬla
separateȱ theȱ i
ngȱwillȱhappe
W 3
abȬ
ironȱ
enȱifȱ
139](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-133-320.jpg)
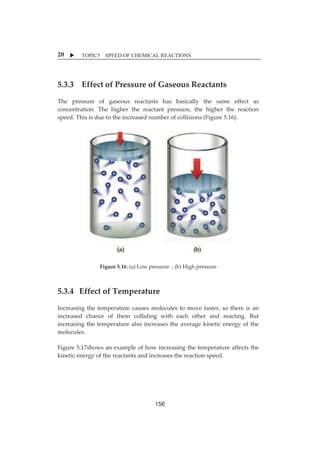
![X TOPIC5 SPEED OF CHEMICAL REACTIONS
4
5.1.2ȱ NatureȱofȱChemicalȱReactantsȱ
Inȱ orderȱ forȱ aȱ reactionȱ toȱ occur,ȱ thereȱ mustȱ beȱ aȱ collisionȱ betweenȱ theȱ
reactantsȱatȱtheȱreactiveȱsiteȱofȱtheȱmoleculeȱwithȱcorrectȱorientationȱandȱitȱ
hasȱ toȱ achieveȱ activationȱ energy.ȱ Thisȱ willȱ leadȱ toȱ effectiveȱ collisionȱ andȱ
chemicalȱreactionȱwillȱoccur.ȱ
ȱ
Figureȱ5.3:ȱParticlesȱshowingȱtheȱeffectiveȱandȱineffectiveȱcollisionȱ
[Source:ȱhttp://2012books.lardbucket.org/books/principlesȬofȬgeneralȬ
chemistryȬv1.0m/s18Ȭ07ȬtheȬcollisionȬmodelȬofȬchemica.html]ȱ
ȱ
Particlesȱmightȱ beȱ atoms,ȱmoleculesȱ orȱ ions.ȱBeforeȱweȱ canȱ getȱ aȱ chemicalȱ
reaction,ȱparticlesȱmustȱcrashȱtogether.ȱTheyȱmustȱcollide.ȱThisȱisȱcalledȱtheȱ
collisionȱtheory.ȱ
ȱȱȱȱȱȱȱȱȱȱȱȱ
Figureȱ5.4:Collisionbetweenȱparticlesȱ
[Source:ȱhttp://minhaji.net/classes/ȱ3107]ȱ
ȱ
140](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-134-320.jpg)







![X T
12
TOPIC5 SPEE
Whereȱ NJȱ
concentra
quantity.ȱ
isȱneeded
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
SEL
ED OF CHEMIC
CAL REACTIO
Averageȱrateȱ=
LF-CHECK 5.
Theȱv
asȱfo
ȱ
Time
Volu
ȱ
i. D
ii. C
.3
volumeȱofȱO2ȱg
llows:ȱ
NS
Brƀ]ȱinitialȱ
nitialȱ
nitialȱ andȱ NJtȱ
ingȱtheȱtimeȱi
ionȱisȱaȱpositi
oȱmakeȱtheȱra
gasȱproducedȱd
eȱ(min)ȱ 0
umeȱ(cm3)ȱ 0
Drawȱaȱgraphȱo
Calculateȱtheȱa
minute.m
ȱ
5.3
Someȱche
toȱspeedȱu
Thereȱareȱ
(a) Part
(b) Con
(c) Pres
(d) Tem
(e) Cata
A
[Brƀ]ȱ =ȱ [Brƀ
ationȱofȱBrƀȱde
Butȱtheȱspeed
ȱinȱtheȱspeedȱ
ȱ 2ȱ
ȱ 60ȱ
ȱ[Brƀ]ȱfinalȱ–ȱ[B
ȱȱȱȱȱȱȱȱtfinalȱ–ȱtȱin
ȬȱNJȱ[Brƀ]ȱ
NJtȱ
ȱ
=ȱ tfinalȱ –ȱ tiniti
interval,ȱNJȱ[Br
iveȱquantity,ȱs
teȱpositive.ȱ
dueȱtoȱtheȱdeco
ofȱtheȱvolumeȱo
averageȱrateȱofȱ
FACTO
AȱREA
emicalȱreactio
upȱtheȱslowȱo
severalȱfacto
ticleȱsizeȱofȱth
ncentrationȱof
ssureȱofȱgaseo
mperature;ȱan
alysts.ȱ
ȱȱȱȱȱȱȱȱȱȱȱȱȱȱȱȱȱ
= ȱ
]finalȱ –ȱ [Brƀ]in
ecreasesȱduri
dȱofȱthereacti
expressionȱto
ORSȱAFF
ACTION
ial.ȱ Becauseȱ t
rƀ]ȱisȱaȱnegati
soȱaȱminusȱsi
ompositionȱofȱH
theȱ
iveȱ
ignȱ
H2O2isȱrecorded
6ȱ 8
82ȱ 84
4ȱ 84ȱ
gainstȱtime.ȱ
theȱrateȱofȱreac
FECTING
nsȱareȱfast;ȱo
onesȱandȱslow
thersȱareȱslow
wȱdownȱtheȱfas
orsȱthatȱaffectȱ
theȱspeedȱofȱa
heȱreactants;ȱ
fȱtheȱreactants
ousȱreactants;
dȱ
s;ȱ
;ȱ
4ȱ
78ȱ
ofȱtheȱO2ȱgasȱa
reactionȱandȱt
dȱ
ctionȱatȱtheȱthir
GȱTHEȱS
10ȱ
rdȱ
SPEEDȱO
w.ȱSometimes
stȱones.ȱ
ȱreaction:ȱ
OFȱ
sȱchemistsȱwa
antȱ
148](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-142-320.jpg)





![X T
18
TOPIC5 SPEE
Sodiumȱth
speedȱtoȱf
equationȱf
ED OF CHEMIC
hiosulphateȱs
formȱaȱyellow
forȱtheȱreactio
CAL REACTIO
olutionȱreacts
wȱprecipitateȱo
onȱis:ȱ
sȱwithȱdiluteȱ
ofȱsulphur,Sȱ(
SO4(aq)ȱȱÆȱȱN
2O3(aq)ȱȱ+ȱȱH2
Na2S2
http://ww
Theȱgraph
a) Gr
ȱ
ȱ
ȱ
ȱ
ȱ
Figureȱ5.1
timeȱ
ȱ
ȱ
Na2SO4(aq)ȱȱ+ȱ
reȱ5.13:ȱTheȱyel
Figur
ww.sciencequi
hȱobtainedȱfro
raphȱofȱconce
Concentration
of sodium
thiosulphate
solution
(mol/dm3)
14:ȱGraphȱofȱc
llowȱprecipitat
[
Source:ȱ
mistry/rates/m
iz.net/lcchem
omȱExperimen
ntȱ5.3ȱshouldȱ
entrationȱofȱso
odiumȱthiosul
Time until
l cross can no long
ger be seen (s)
nȱofȱsodiumȱth
concentration
NS
sulphuricȱaci
Figureȱ5.8).ȱT
dȱatȱaȱveryȱlo
Theȱchemicalȱ
ȱS(s)ȱȱ+ȱȱSO2(g
ȱ
eȱofȱsulphurȱȱ
g)ȱȱ+ȱȱH2O(l)ȱ
matching/conc/
owȱ
m]ȱ
/rate_conc.htm
beȱshownȱasȱ
follows:ȱ
lphateȱsolutio
onȱagainstȱtim
ȱ
hiosulphateȱso
meȱ
olutionȱagain
stȱ
154](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-148-320.jpg)

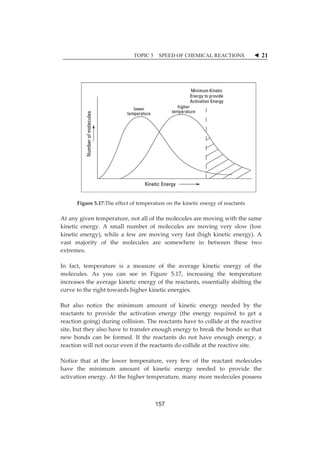





![TOPIC 5 SPEED OF CHEMICAL REACTIONS W 27
THEȱEFFECTȱOFȱACTIVATIONȱ
ENERGYȱONȱTHEȱSPEEDȱOFȱAȱ
REACTIONȱ
5.4
Allȱmoleculesȱpossessȱaȱcertainȱminimumȱamountȱofȱenergy.ȱTheȱenergyȱcanȱ
beȱ inȱ theȱ formȱ ofȱ kineticȱ energyȱ and/orȱpotentialȱ energy.ȱWhenȱmoleculesȱ
collide,ȱtheȱkineticȱenergyȱofȱtheȱmoleculesȱcanȱbeȱusedȱtoȱstretch,ȱbendȱandȱ
ultimatelyȱbreakȱtheȱbonds,ȱleadingȱtoȱchemicalȱreactions.ȱȱ
Ifȱmoleculesȱ areȱ movingȱ tooȱ slowlyȱwithȱ littleȱ kineticȱ energy,ȱ orȱ collidedȱ
withȱanȱimproperȱorientation,ȱtheyȱwillȱnotȱreactȱandȱsimplyȱbounceȱoffȱeachȱ
other.ȱHowever,ȱifȱtheȱmoleculesȱareȱmovingȱatȱaȱfastȱenoughȱvelocityȱwithȱaȱ
properȱ collisionȱ orientation,ȱ suchȱ asȱ theȱ kineticȱ energyȱ uponȱ collisionȱ isȱ
greaterȱ thanȱ theȱminimumȱ energyȱ barrier,ȱ thenȱ aȱ reactionȱwillȱ occur.ȱ Theȱ
minimumȱenergyȱbarrierȱthatȱmustȱbeȱmetȱforȱaȱchemicalȱreactionȱtoȱhappenȱ
isȱcalledȱtheȱactivationȱenergy,ȱEa.ȱItȱcanȱbeȱrepresentedȱbyȱtryingȱtoȱpushȱaȱ
stoneȱtoȱtheȱotherȱsideȱasȱshownȱinȱFigureȱ5.22.ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
ȱ
Figureȱ5.23:ȱTheȱmanȱisȱtryingȱtoȱpushȱtheȱstoneȱfromȱpointȱAȱtoȱpointȱBȱ
[Source:ȱhttp://sites.tenafly.k12.nj.us/~shilfstein/demo_notes.htm]ȱ
Theȱ reactionȱ pathwayȱ canȱ beȱ observedȱ inȱ Figureȱ 5.23.ȱ Inȱ orderȱ toȱ getȱ theȱ
productȱ toȱ react,ȱ theȱ reactantȱ hasȱ toȱ overcomeȱ theȱ activationȱ energy,ȱ orȱ aȱ
newȱ productȱ cannotȱ beȱ achievedȱ ifȱ itȱ doesȱ notȱ haveȱ theȱ sameȱ amountȱ ofȱ
energy.ȱ
163](https://image.slidesharecdn.com/hbsc3203kimiaii-141006190535-conversion-gate01/85/HBSC-3203-157-320.jpg)