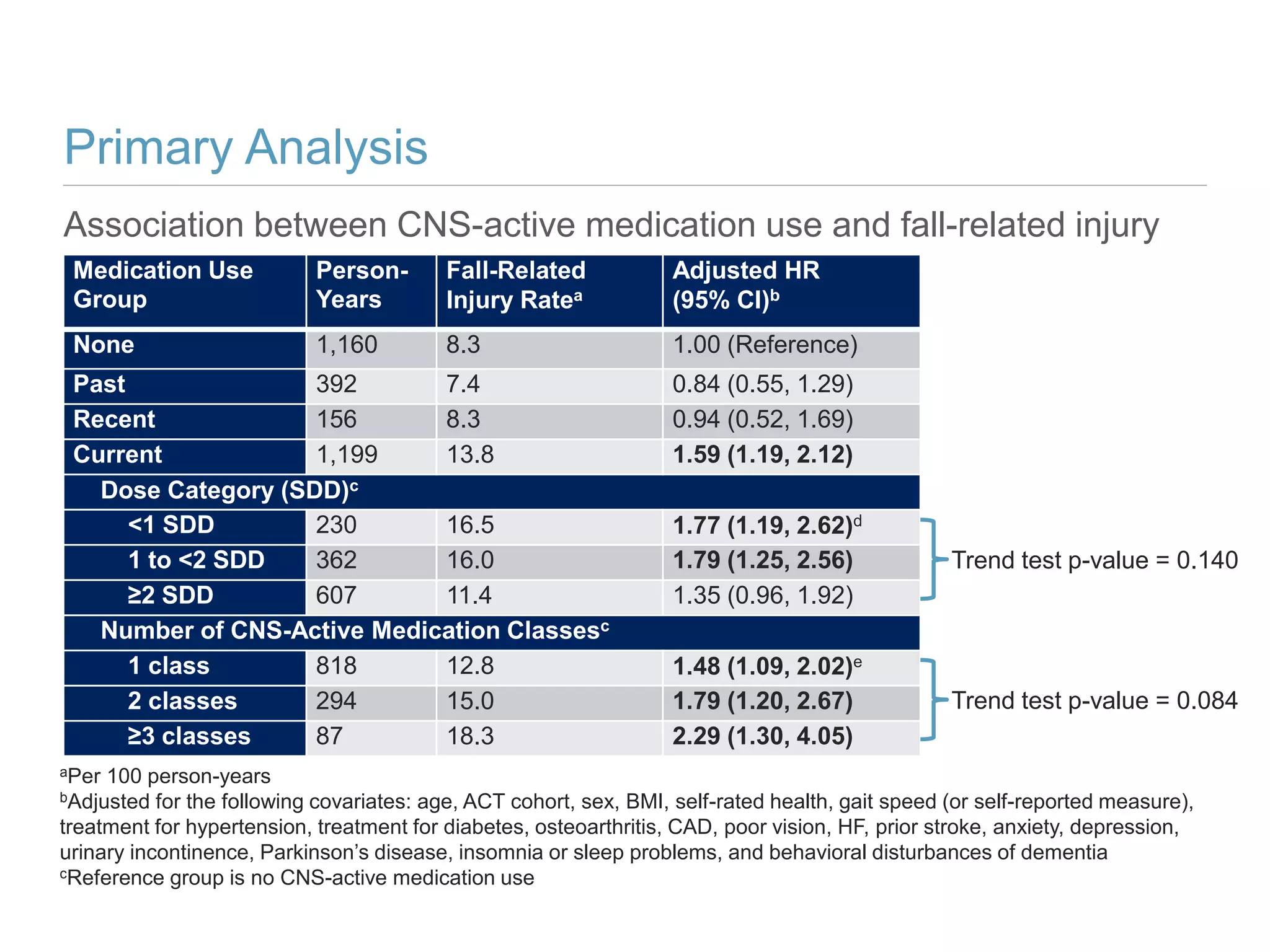
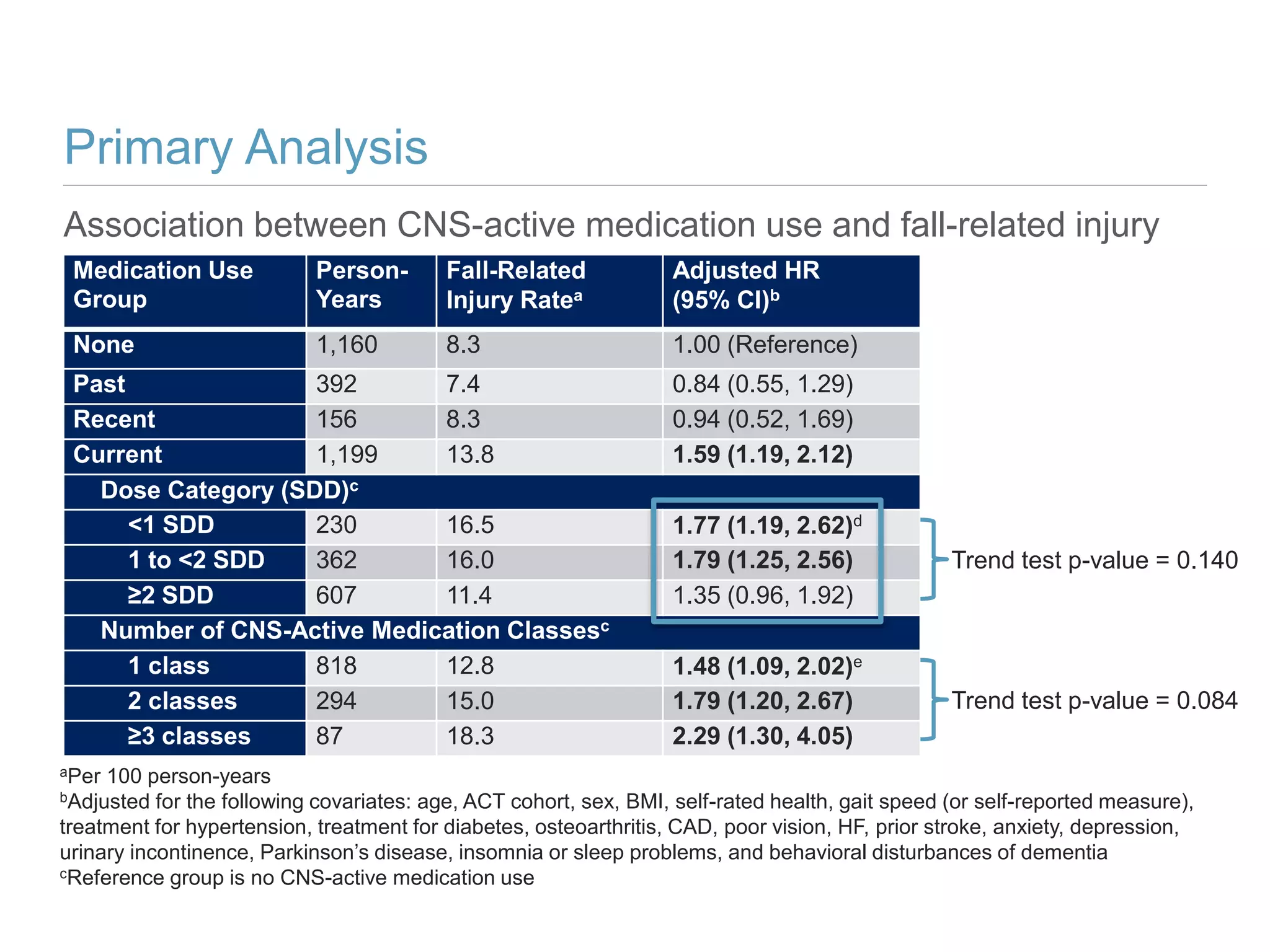
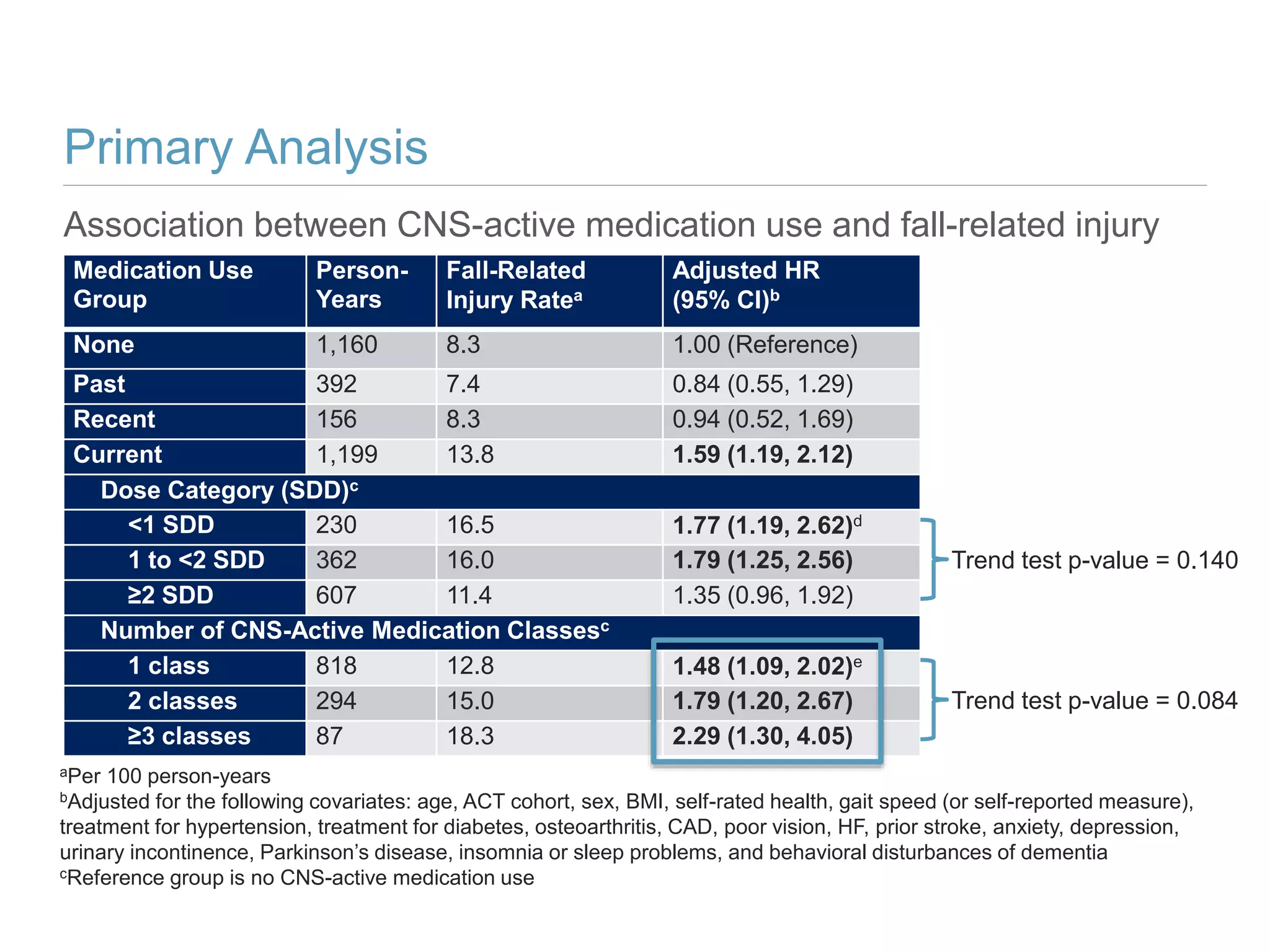
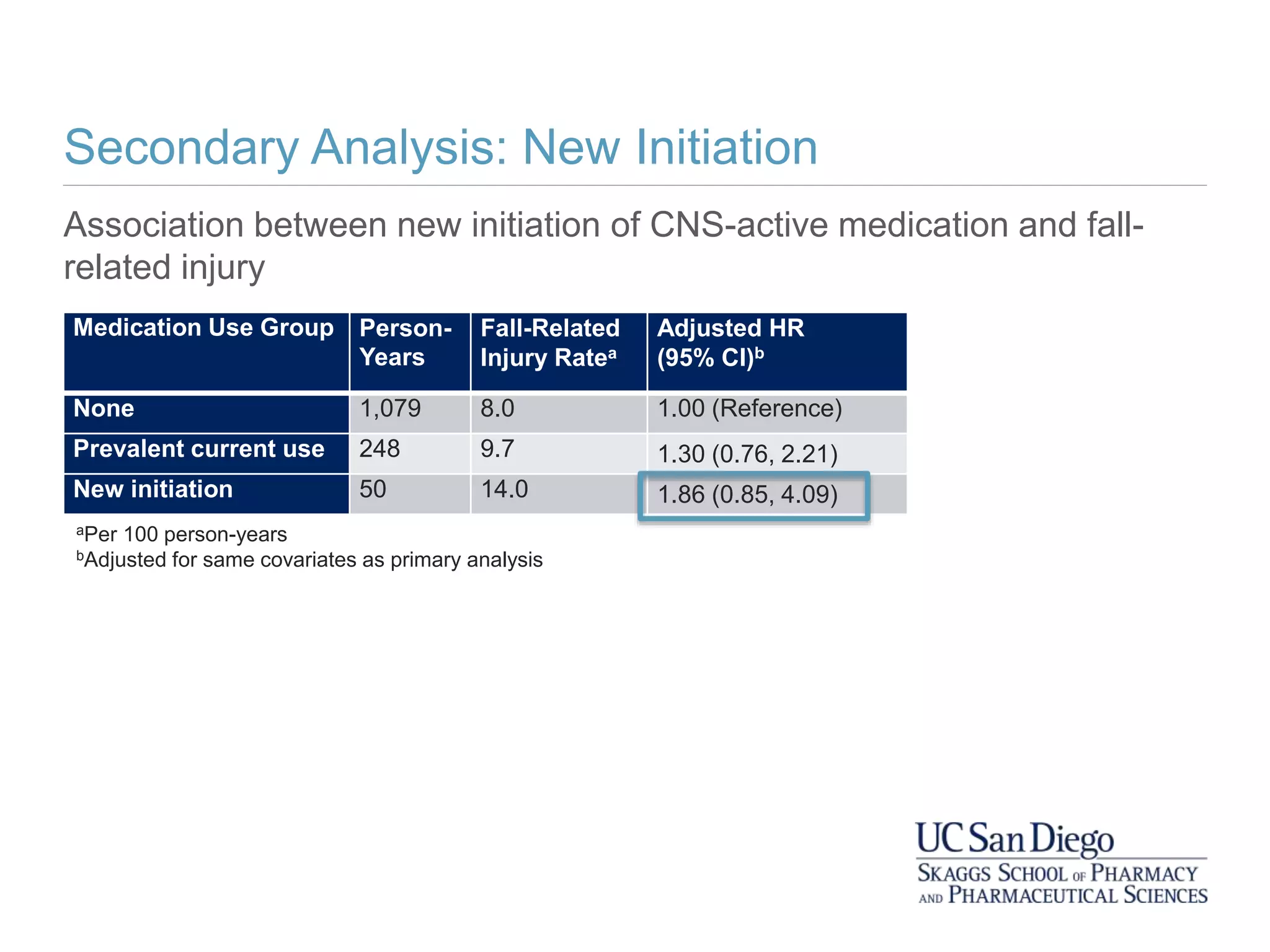
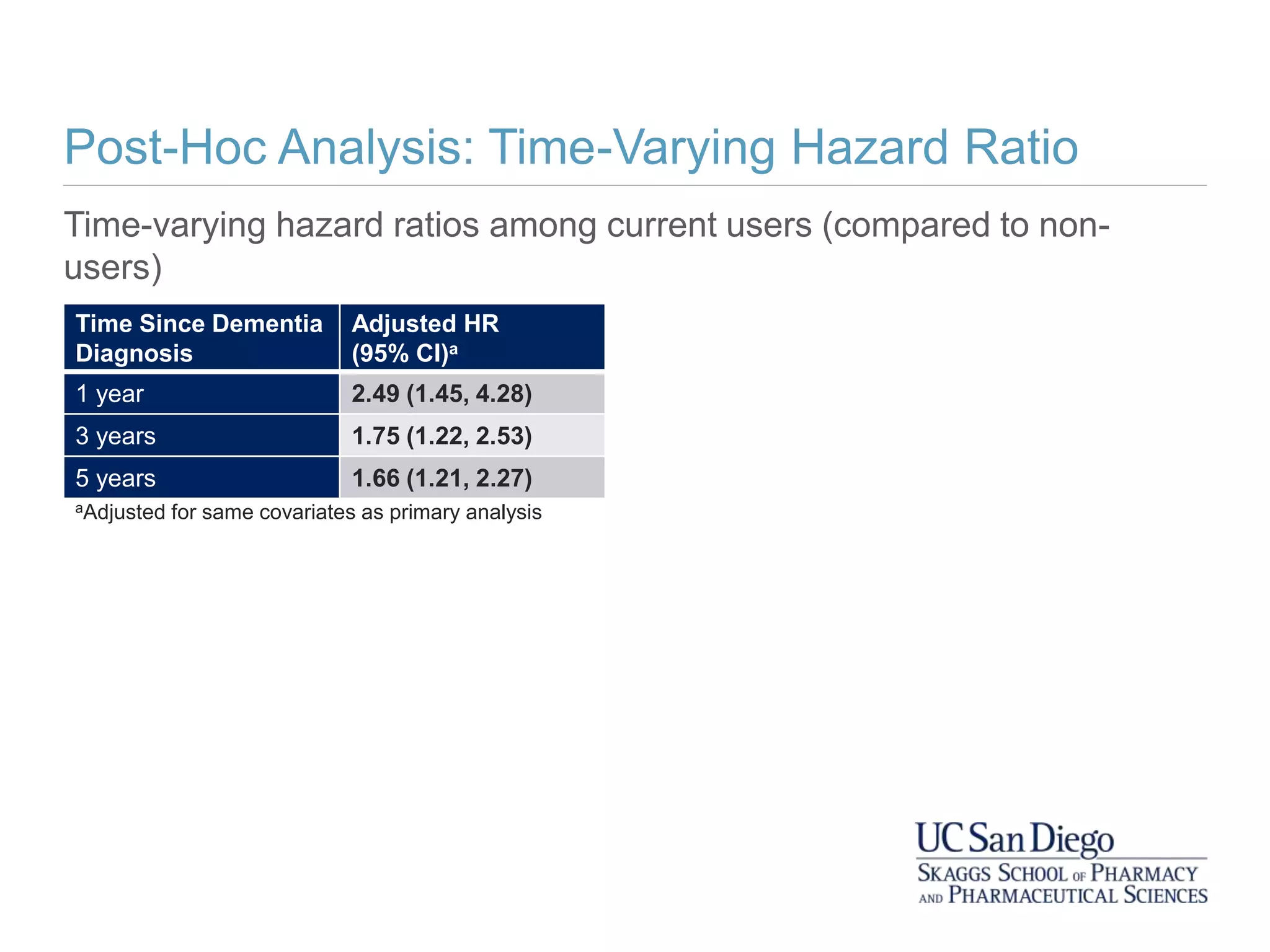
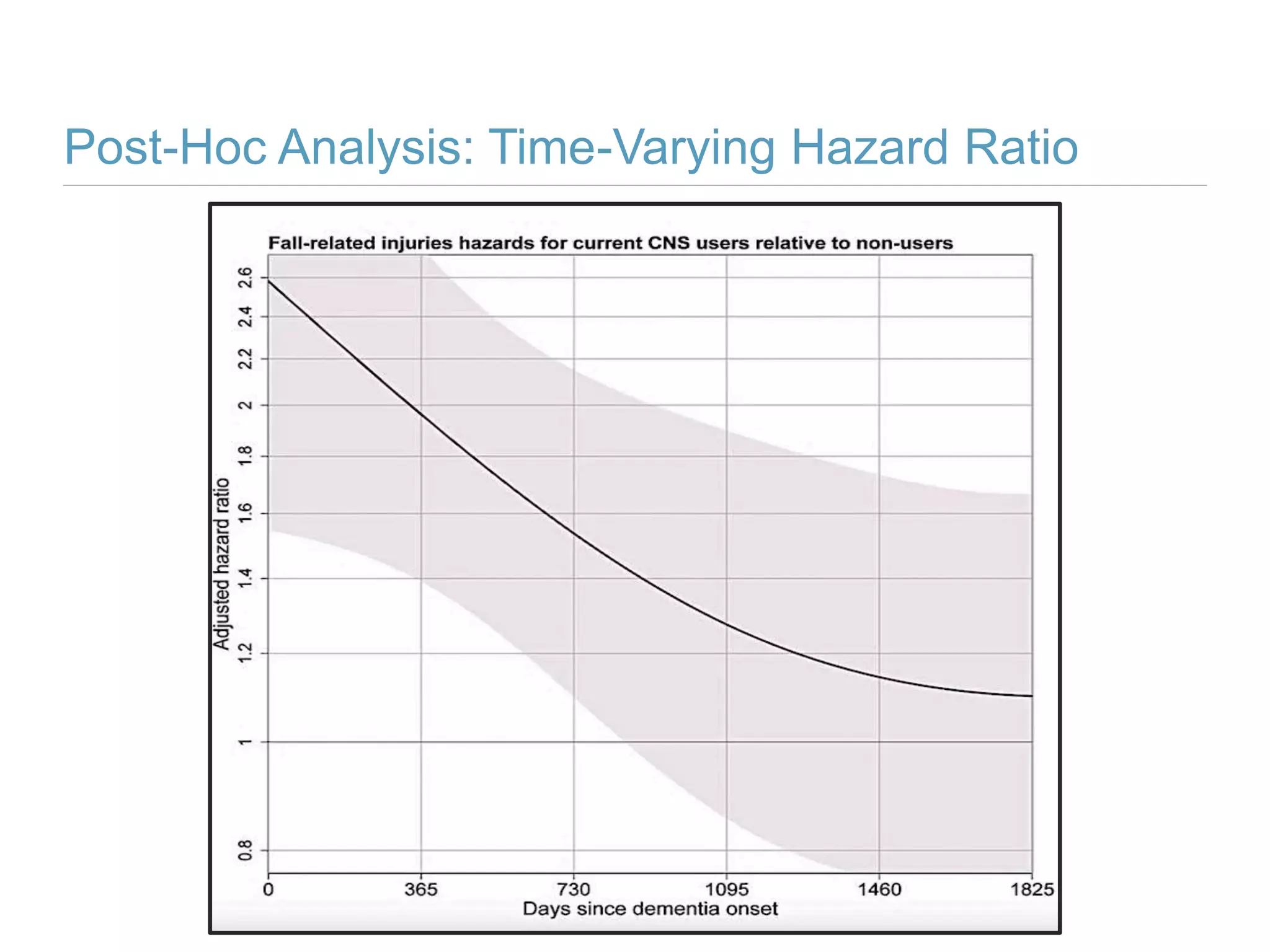
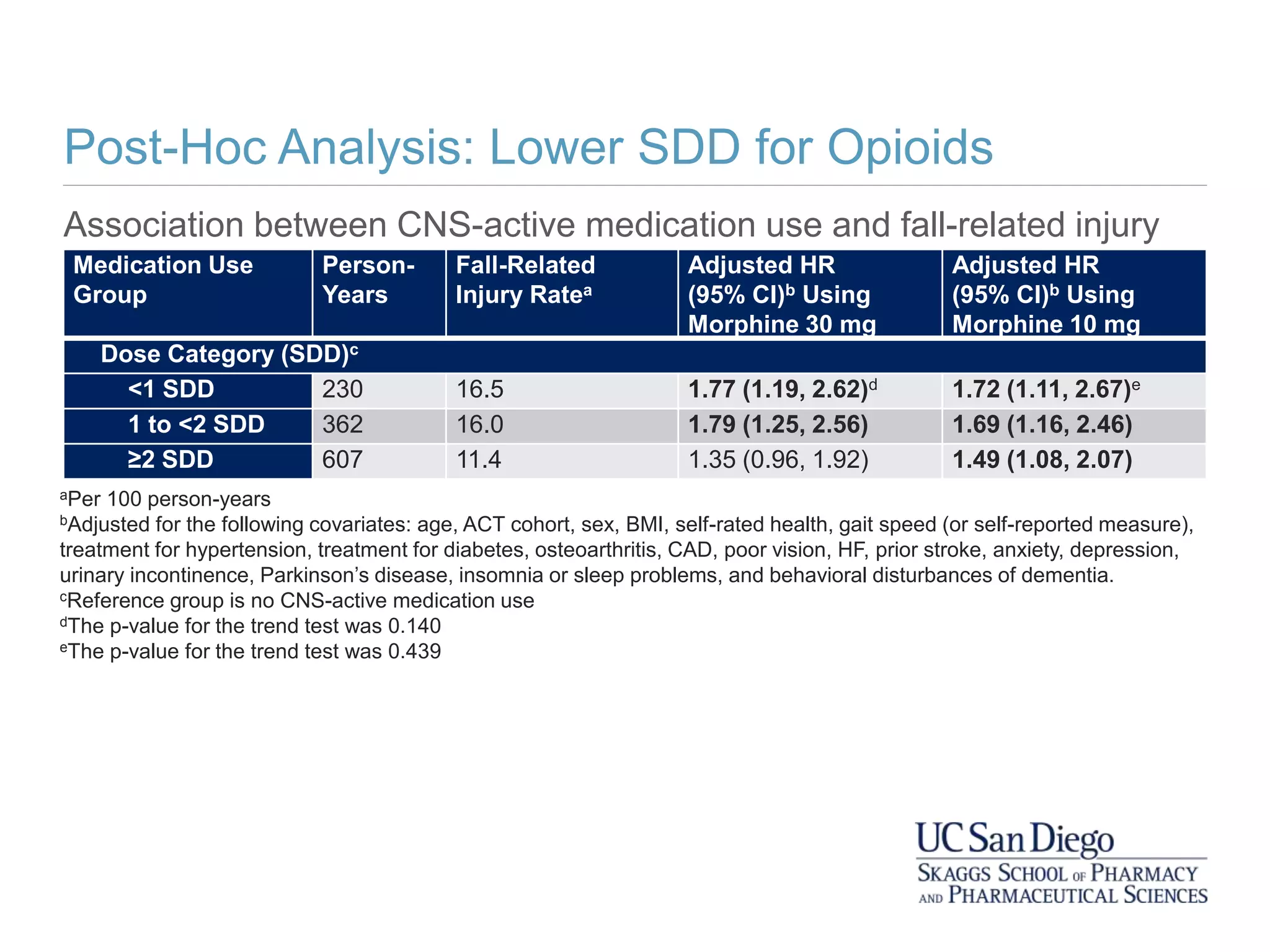
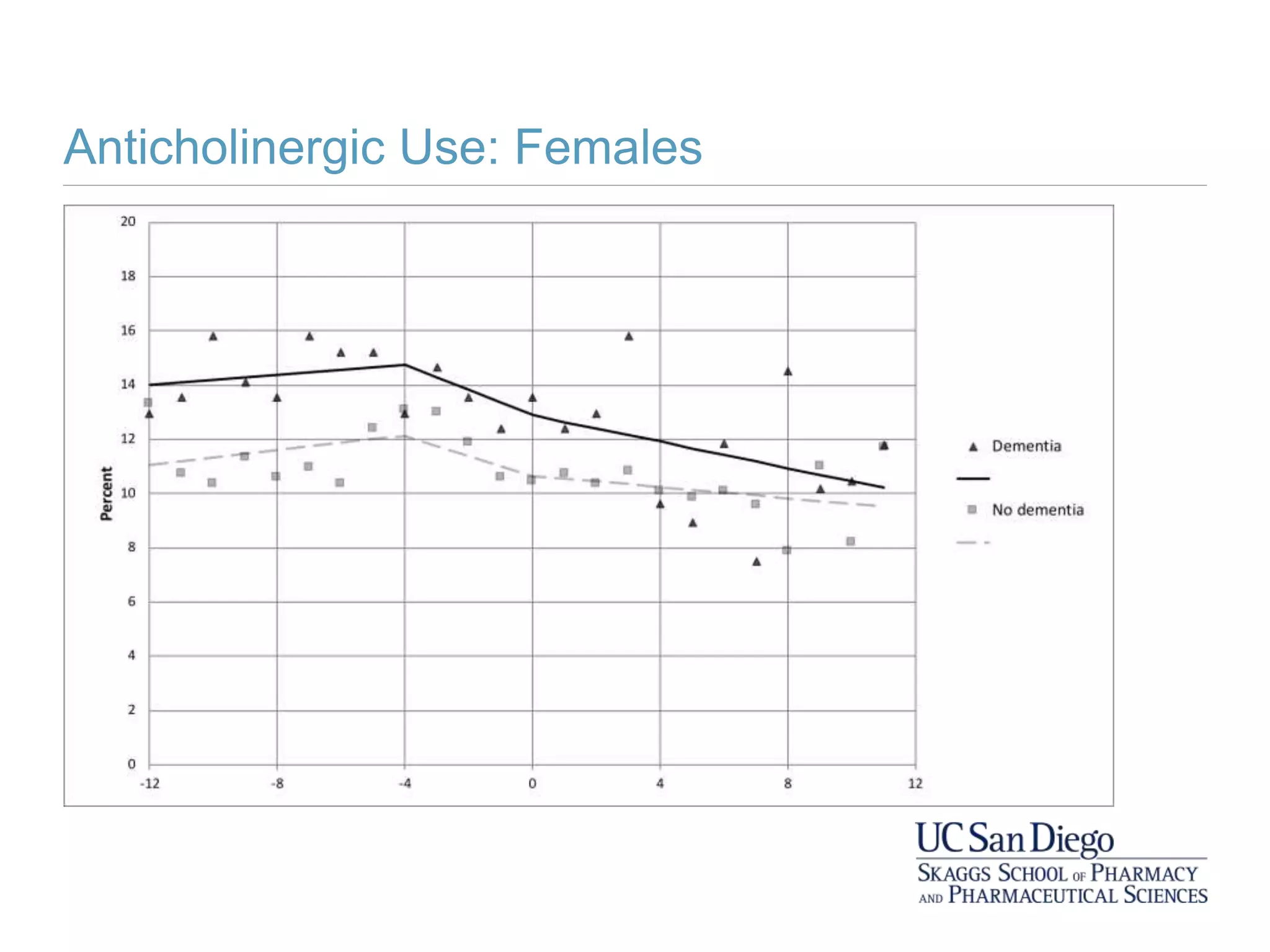
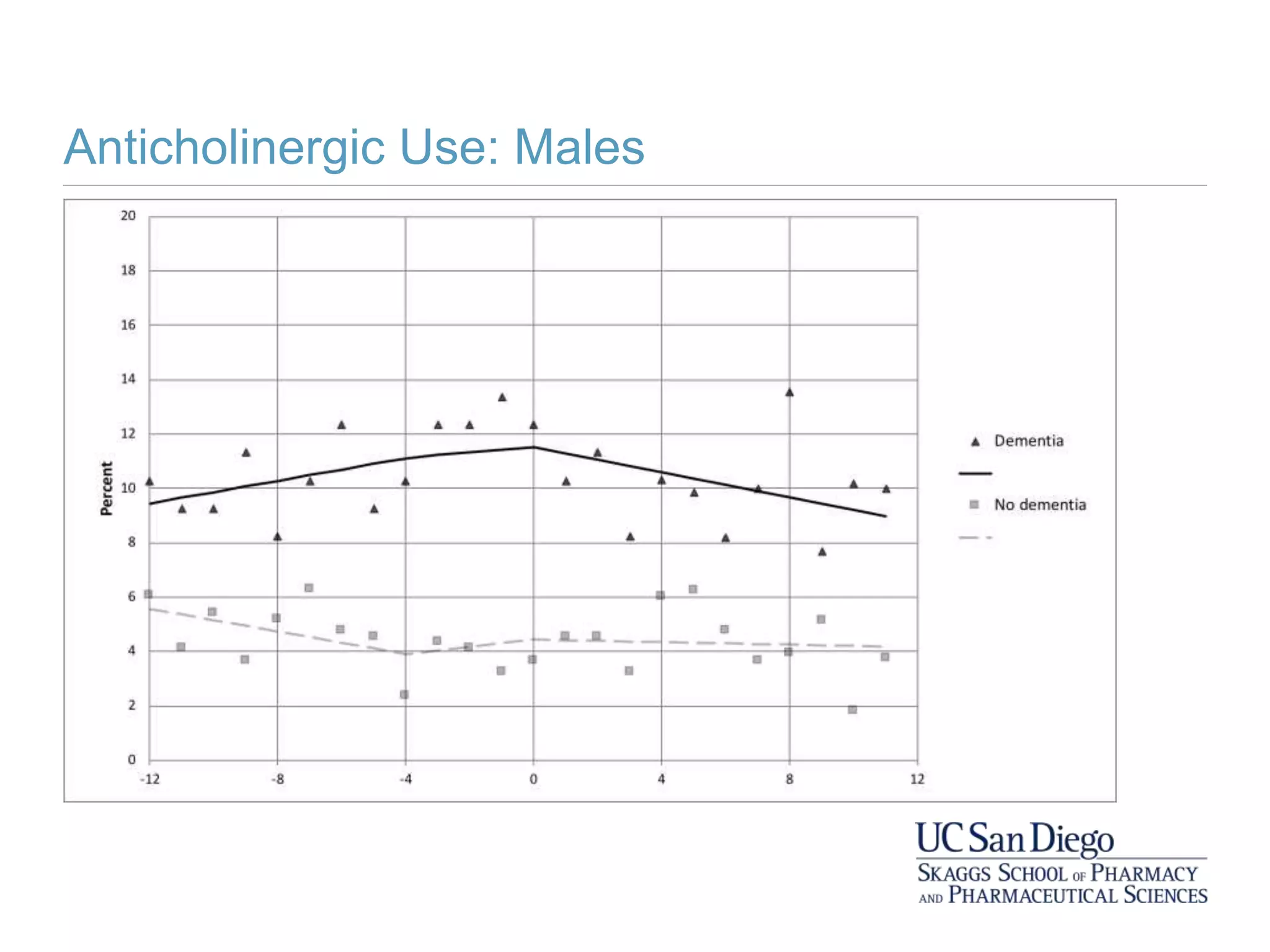
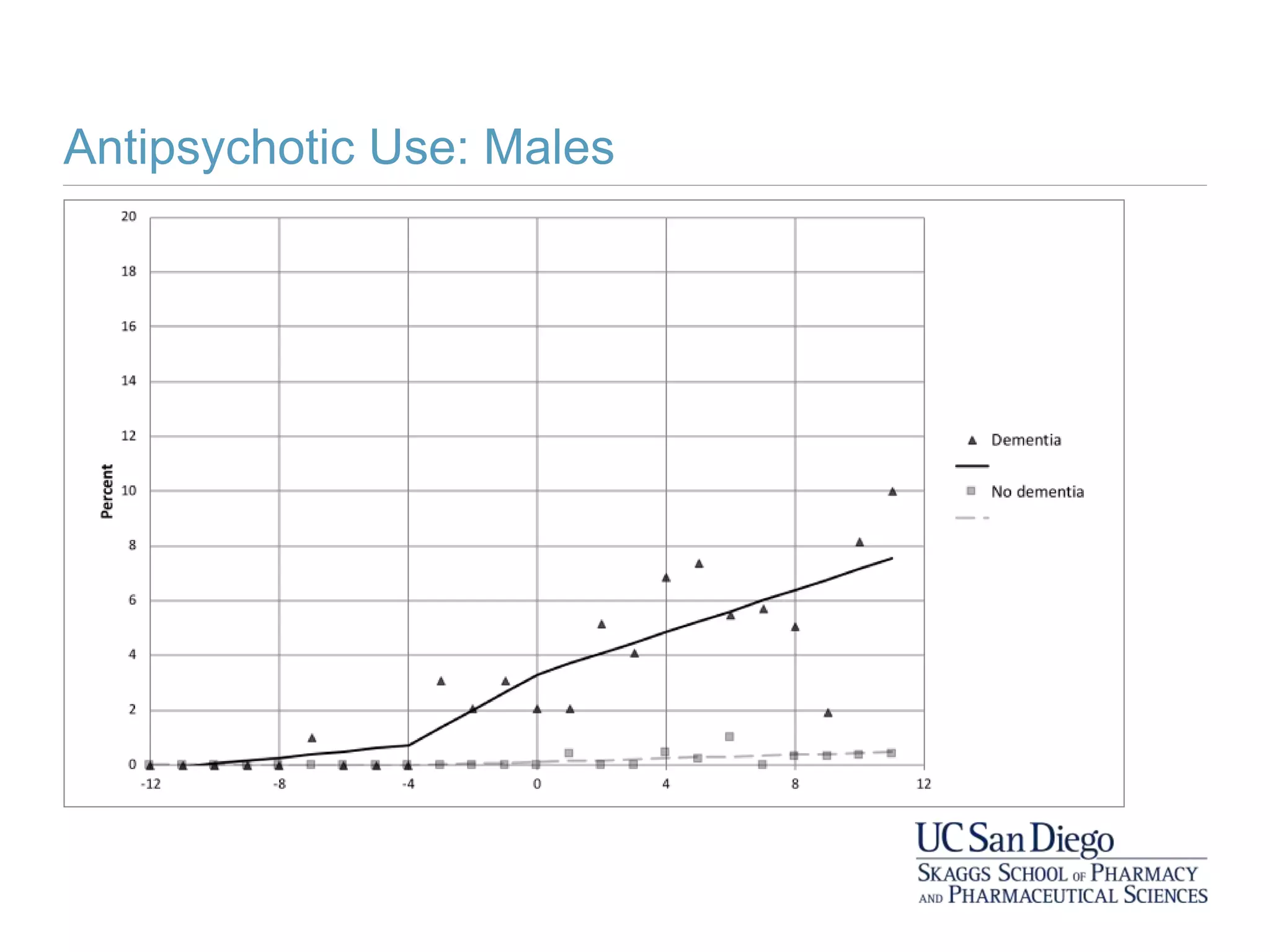
This study investigates the association between the use of central nervous system (CNS) active medications and the risk of fall-related injuries in community-dwelling older adults recently diagnosed with dementia. The findings indicate that current use of CNS-active medications significantly increases the risk of falls, particularly in those using higher doses or multiple classes of these medications. The research highlights the need for careful medication management in this vulnerable population to prevent fall-related injuries.





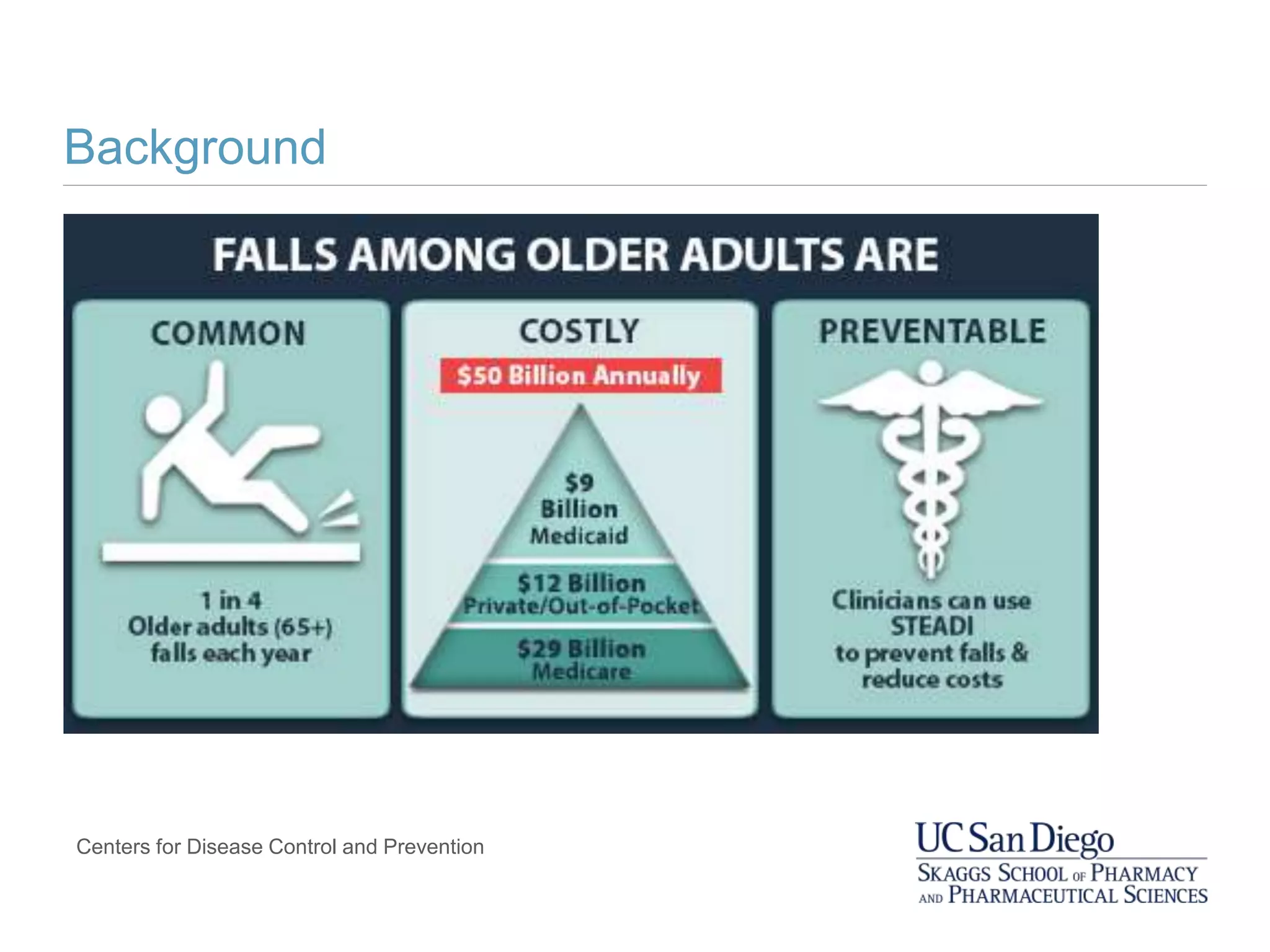
![Falls in Older Adults with Dementia
In older adults with dementia, compared to those without
dementia:
• The risk of falling is higher (estimates of 2-8x)
• Health outcomes resulting from a fall are even more
devastating
Van Doorn C et al. J Am Geriatr Soc. 2003;51:1213-1218.
Tinetti ME et al. N Engl J Med. 1988;319(26):1701-7.
Magaziner J et al. J Gerontol. 1990;45(3):M101-7.
Meuleners LB et al. J Am Geriatr Soc. 2017;65(3):520-525.
Allan L et al. PloS one. 2009;4[5]:e5521](https://image.slidesharecdn.com/hartgweppresentationslides-190414234018/75/Hart-gwep-presentation-slides-7-2048.jpg)