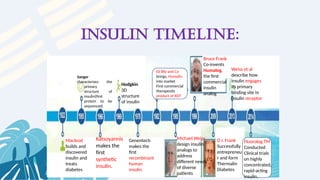
The document provides a comprehensive overview of human insulin, detailing its history, types, production techniques, and the advancements in recombinant DNA technology for insulin production. It highlights the transition from animal-derived insulin to genetically engineered insulin using E. coli and yeast, emphasizing the advantages of recombinant approaches and advanced purification methods. Additionally, it discusses the significance of insulin in diabetes management and the projected rise in diabetes cases globally.