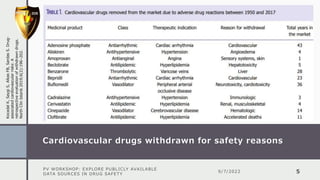
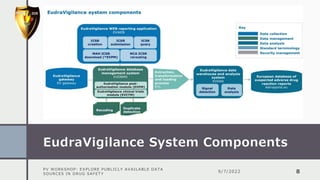
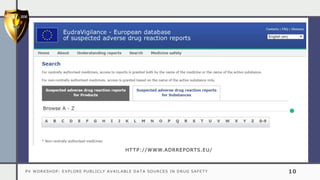
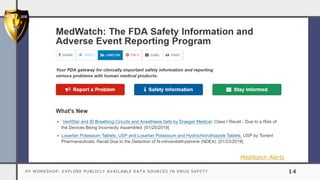
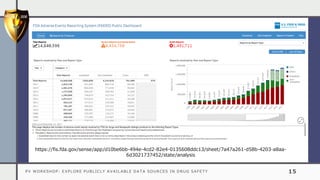
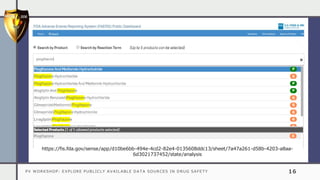
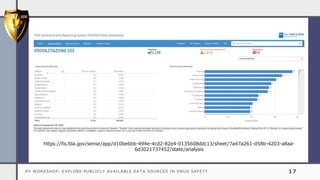
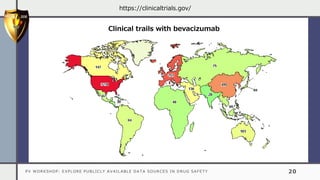
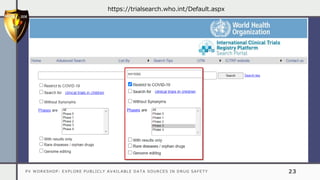
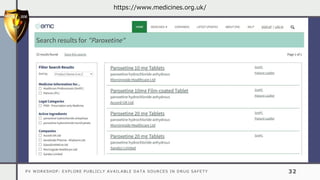
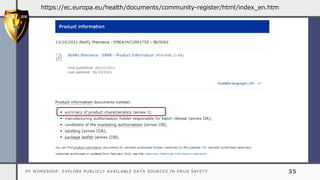
The document details a pharmacovigilance workshop focused on exploring publicly available data sources related to drug safety. It discusses significant safety withdrawals of medicines due to adverse effects not detected in clinical trials, as well as various information sources such as clinical trial registries and databases for drug safety surveillance in the USA and Europe. Additionally, it provides links to important resources for product authorization and labeling, aiming to enhance understanding of drug safety mechanisms.