The document outlines updates to Module IX of the GVP including shorter timelines for informing health authorities of emerging safety issues, submitting updates to product information or risk management plans within 3 months of signal validation, and reviewing EudraVigilance data more frequently. It indicates major potential impacts including setting up processes to meet new timelines and requirements as well as training personnel and managing resources accordingly.



![IX.C.3 – Procedure for validated signals
• IX.C.3.1 – Emerging safety issues (ESI)
▫ Updated definition for ESI – “A safety issue…[with a]…
potential major impact on the risk-benefit balances of the
product and/or on patient or public health, that could
warrant prompt regulatory action and communication to
patients and healthcare professionals.”
• In case of an ESI, Health Authorities are to be informed
within two days of validation
• Potential Impact – Major
▫ Process update to ensure timely information delivery to HA
for ESI
▫ Process update to incorporate the shortened timelines
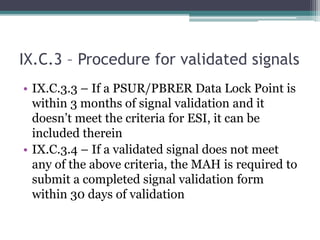
▫ Information dissemination to concerned functions](https://image.slidesharecdn.com/gvpmoduleixrev1andaddendum1-170520160945/85/GVP-Module-IX-rev-1-5-320.jpg)