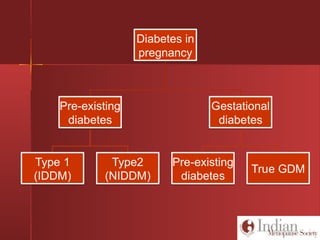
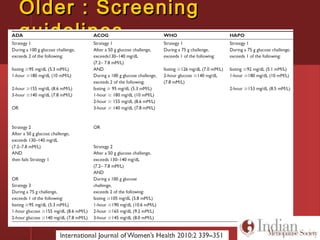
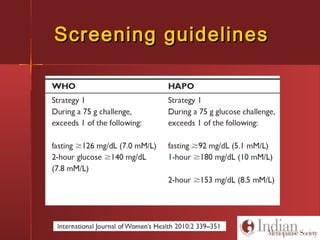
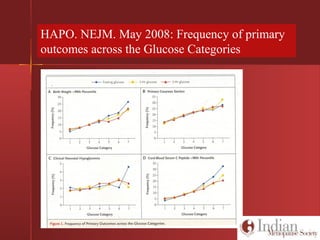
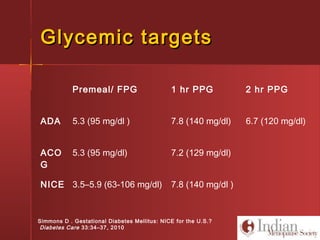
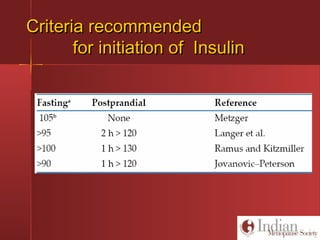
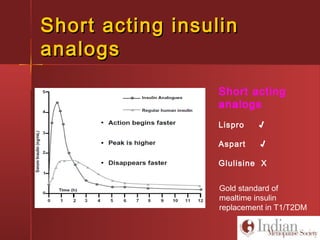
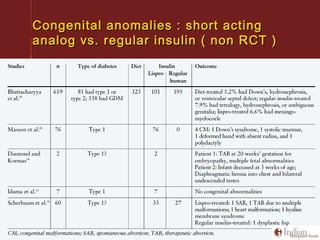
This document discusses gestational diabetes, including controversies around screening and diagnosis, management through diet, medication and insulin, and safety considerations. It provides an overview of gestational diabetes, risk factors, epidemiology in India, current screening guidelines, treatment options including medical nutrition therapy, oral medications and insulin regimens, and goals for glucose control. Safety of different insulin analogs is also reviewed, noting minimal transfer of lispro and aspart across the placenta and no increased risk of congenital anomalies.