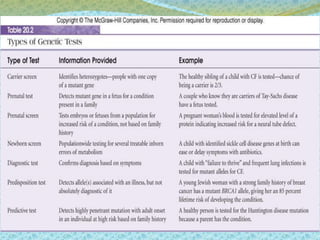
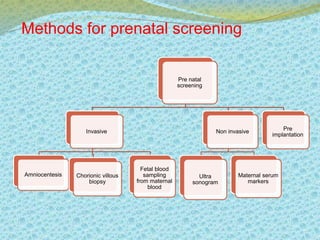
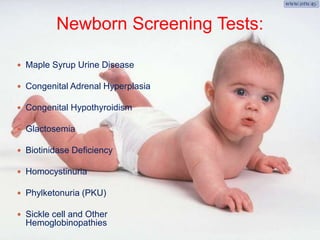
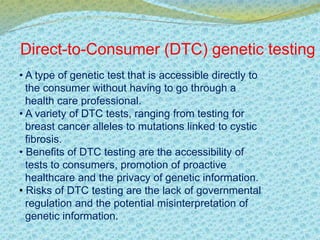
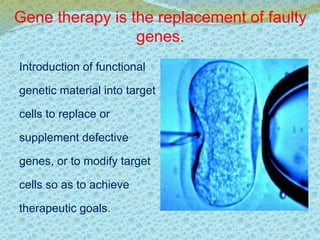
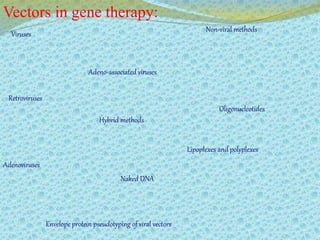
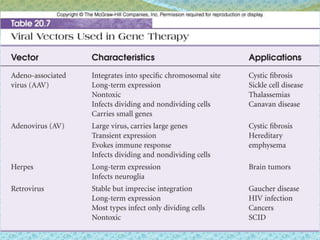
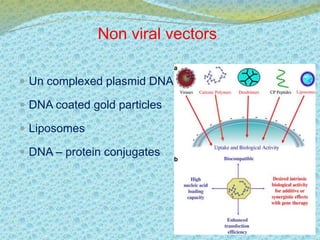
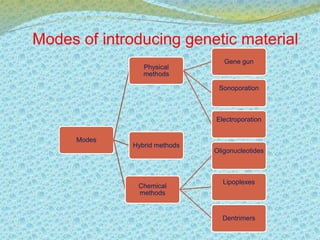
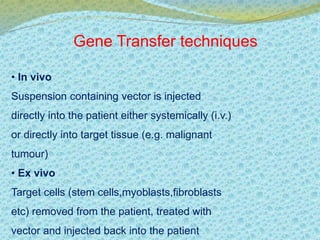
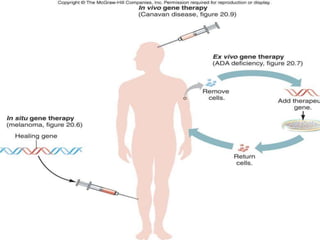
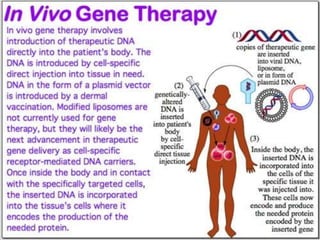
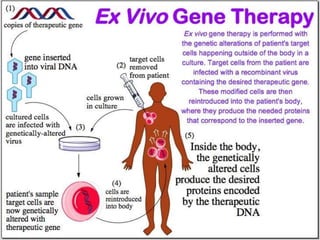
This document discusses the history and current state of genetic screening, genetic counseling, and gene therapy. It provides a timeline of major developments in these fields from 1961 to 2008. Key points include the first approved gene therapy case in 1990, the first gene therapy treatment for ADA deficiency in 2002, and over 1,200 genetic tests being available by 2008. The document also describes various genetic screening tests, the role of genetic counseling, methods of genetic screening and gene therapy delivery, as well as challenges and applications of these emerging fields.