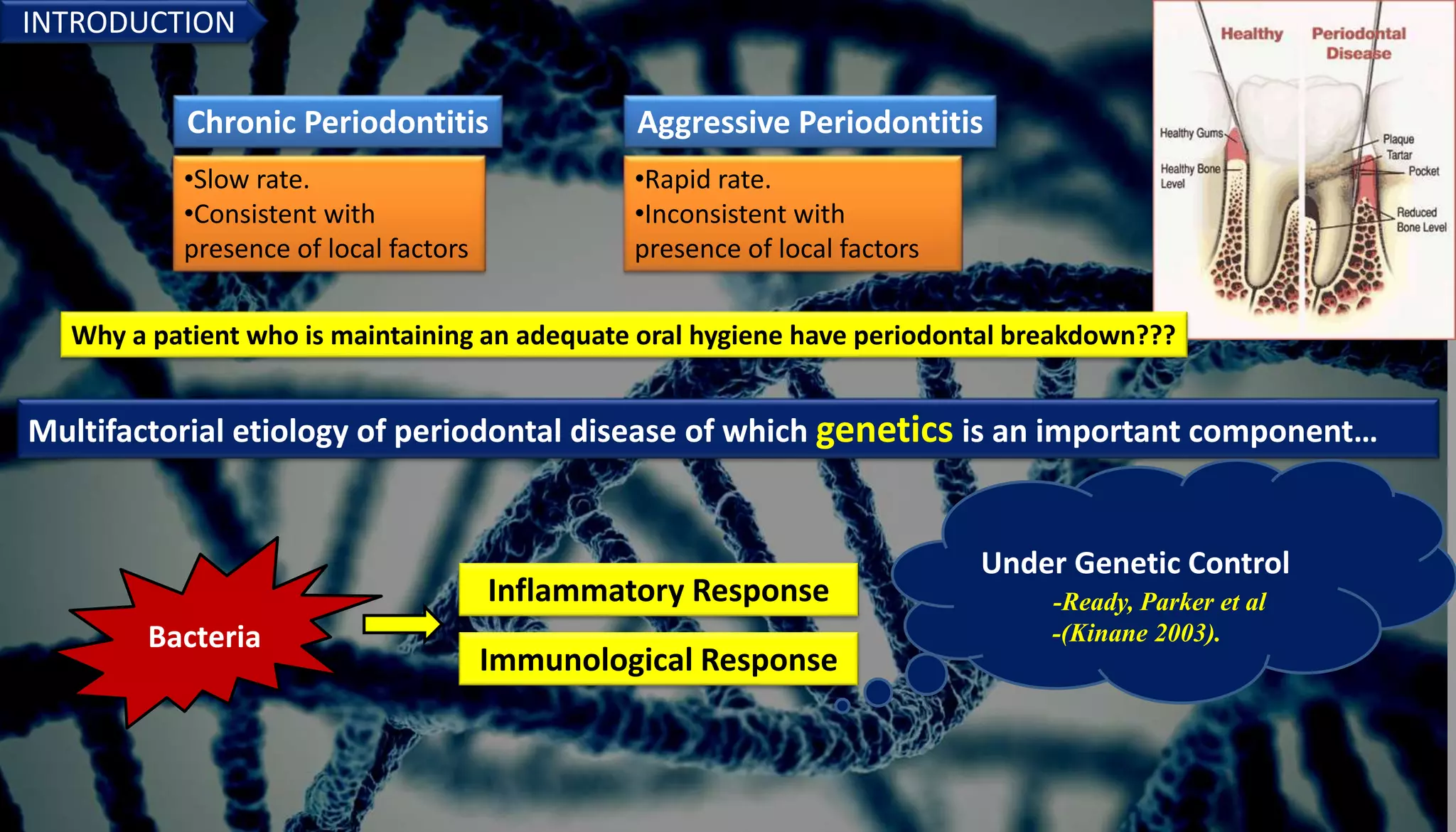
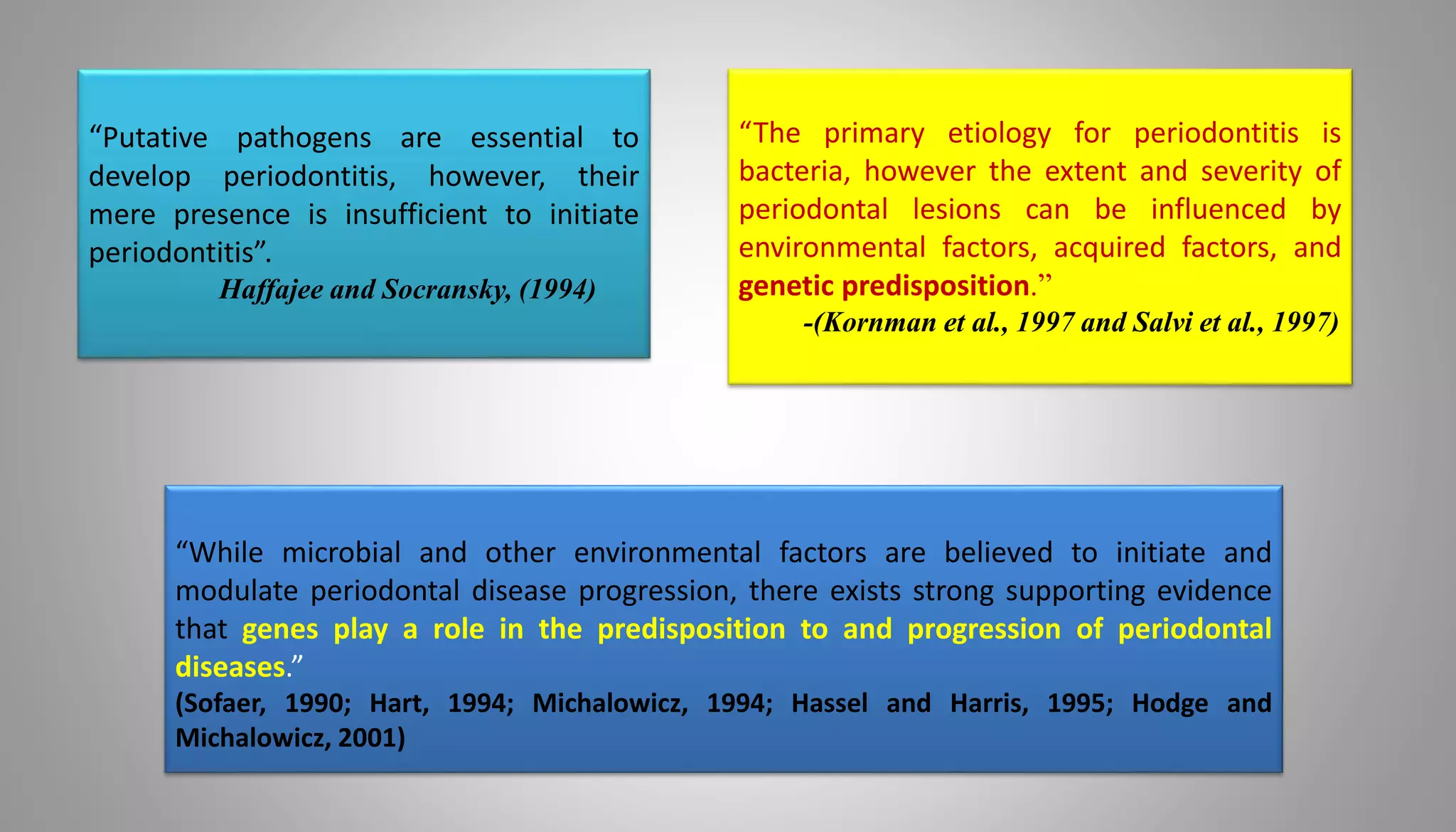
Genetics play an important role in periodontal diseases. The document discusses how genetics and environmental factors interact to determine disease severity and progression. Twin studies estimate that genetics account for 40-80% of chronic periodontitis risk, indicating a strong hereditary component. Several genes have been associated with increased risk of chronic and aggressive periodontitis, including genes related to the inflammatory response. Understanding the genetic factors involved can provide insights into disease pathogenesis and potential targets for risk assessment and treatment.


















































![• The Gm allotype genes, or genes in linkage equilibrium with
them, appear to influence expression of the IgG2 molecule.
• This response appears to be race specific, and young
Caucasians of the low-responder phenotype G2m(null)
[G2m("), G2m(-n) and G2m(-23)]are predisposed to specific
bacterial infections.
Choi et al. (1996) :
The rapidly progressive periodontitis patients who
were positive for the G2m(23) allotype had
elevated antibody to Porphyromonas gingivalis .
In addition to host factors, environmental factors are
capable of modulating IgG2 response. Cigarette
smoking is known to reduce serum IgG levels.](https://image.slidesharecdn.com/geneticsinperiodonticssemiar-230506202033-b560b643/75/GENETICS-IN-PERIODONTICS-SEMIAR-pptx-54-2048.jpg)
























![FELTY'S SYNDROME
Felty's syndrome, is characterized by the combination of rheumatoid
arthritis, splenomegaly and neutropenia
The cause of Felty's syndrome is unknown.
It is more common in people who have had rheumatoid arthritis for a long
time.
People with this syndrome are at risk of infection because they have a low
white blood cell count.
wide spread and early periodontal tissue destruction[aggressive
periodontitis].](https://image.slidesharecdn.com/geneticsinperiodonticssemiar-230506202033-b560b643/75/GENETICS-IN-PERIODONTICS-SEMIAR-pptx-79-2048.jpg)










