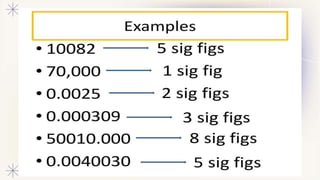
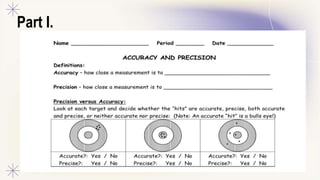
This document discusses accuracy, precision, and significant figures in measurement. It defines accuracy as how close a measurement is to the true value, and precision as how close repeated measurements are to each other. It then explains that significant figures refer to the useful digits in a measurement when written in scientific notation. The document outlines some key rules for determining the number of significant figures in a measurement, such as non-zero digits being significant but leading zeroes not, and zeroes between numbers or after a decimal being significant. It encourages practicing applying these rules to measurements.