This document provides information about isotopes, including:
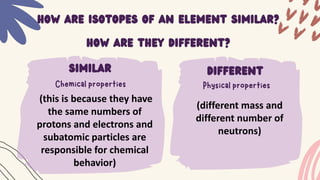
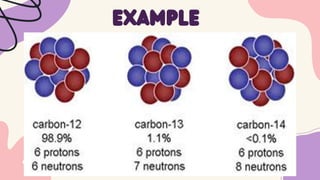
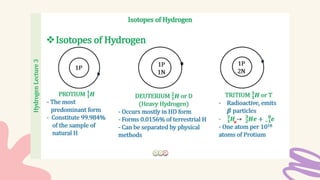
1. Isotopes of an element differ in the number of neutrons but have the same number of protons and electrons, making their chemical behaviors similar.
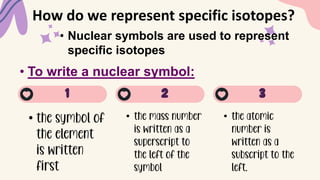
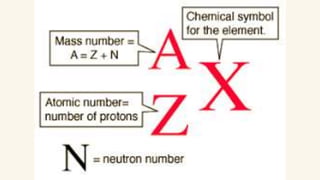
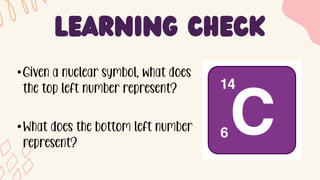
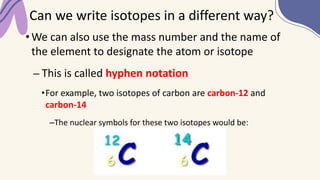
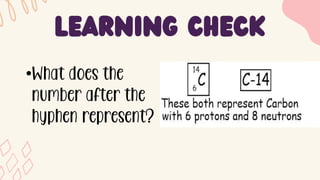
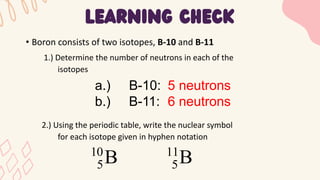
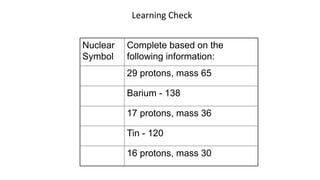
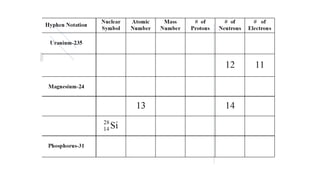
2. Specific isotopes are represented by nuclear symbols noting the element symbol, mass number superscript left of the symbol, and atomic number subscript left of the symbol.
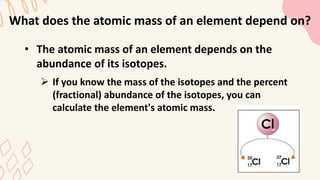
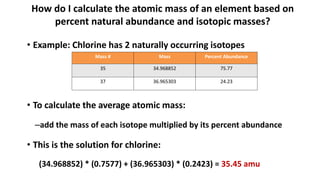
3. The atomic mass of an element depends on the relative abundance of its isotopes, calculated by multiplying each isotope's mass by its natural percentage and totaling.