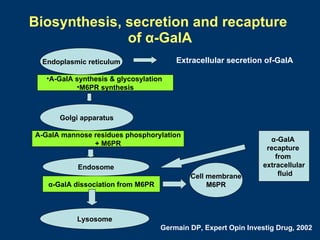
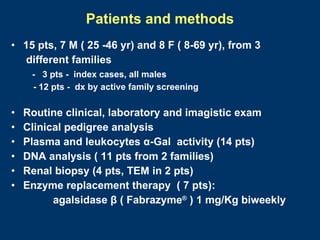
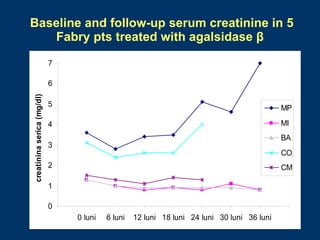
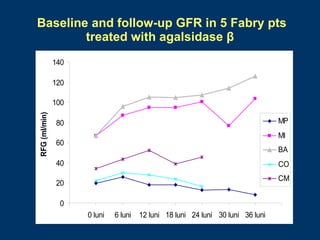
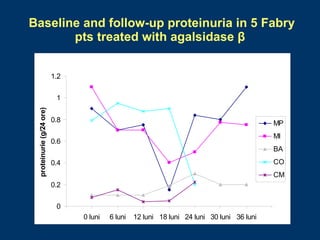
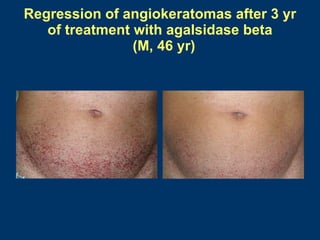
Fabry disease patients were treated with agalsidase beta enzyme replacement therapy. Over 3 years:
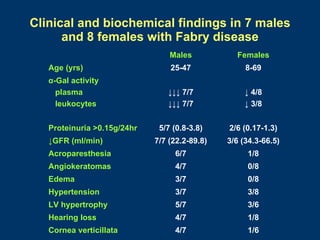
- Renal function stabilized or improved in 5 patients based on serum creatinine and GFR measurements. Proteinuria also decreased.
- Non-renal symptoms like left ventricular hypertrophy, neuropathic pain, and skin lesions improved in several patients. Quality of life increased in most patients.
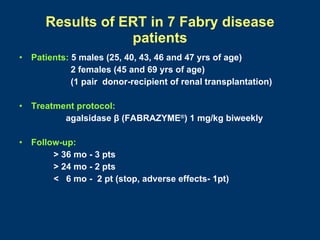
- No major adverse events occurred during treatment. Enzyme replacement therapy with agalsidase beta showed benefits for renal and extrarenal symptoms in Fabry disease patients.