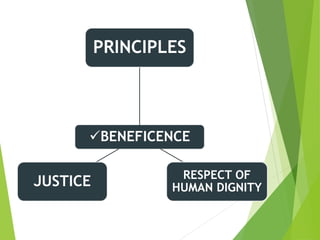
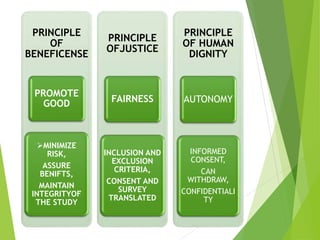
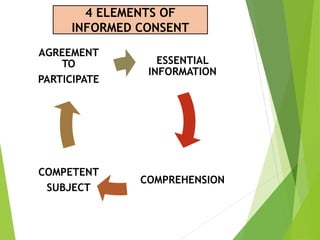
This document discusses ethics in nursing research. It defines ethics in nursing research as the moral principles researchers must follow to ensure the rights and welfare of study participants. It emphasizes protecting vulnerable groups from harm, safeguarding participants from exploitation, establishing risk-benefit ratios, and ensuring respect, dignity, privacy and informed consent. The key ethical principles of beneficence, respect for human dignity and justice are outlined. Guidelines for informed consent including essential elements and from organizations like ICMR are provided. The Code of Ethics for Nurses in India regarding respecting individuals and maintaining competence, boundaries, teamwork and societal trust is also summarized.