This document discusses enterocutaneous fistulas, including:
1. Fistulas are abnormal connections between two epithelial surfaces, most commonly occurring after abdominal surgery as a complication.
2. Classification systems include etiologic (cause), anatomic (location), and physiologic (output amount) which provide understanding for management.
3. Prevention focuses on proper preoperative patient preparation and surgical technique to reduce postoperative fistula risks.

![Introduction
Fistula is derived from Latin word that means “PIPE”.
A Fistula is an abnormal connection between two epithelised surfaces.
Fistulas that involve Gut and Skin are called ENTEROCUTANEOUS
FISTULA.
Most GI Fistula (75 % - 85 %) occur as a complication of abdominal
surgery . However 15 – 25 % of fistula evolve spontaneously [1].
1. www.emedicine.medscape.com/article/intestinalfistula](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-2-2048.jpg)
![Introduction
Even with recent advances in management & critical care
enterocutaneous fistulas remain great challenges to the
surgeon.
Mortality remains high, between 10–30% in recent series,
largely due to the frequent complications of sepsis and
malnutrition.[1]
1.Ann Surg 1960;152:445](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-3-2048.jpg)



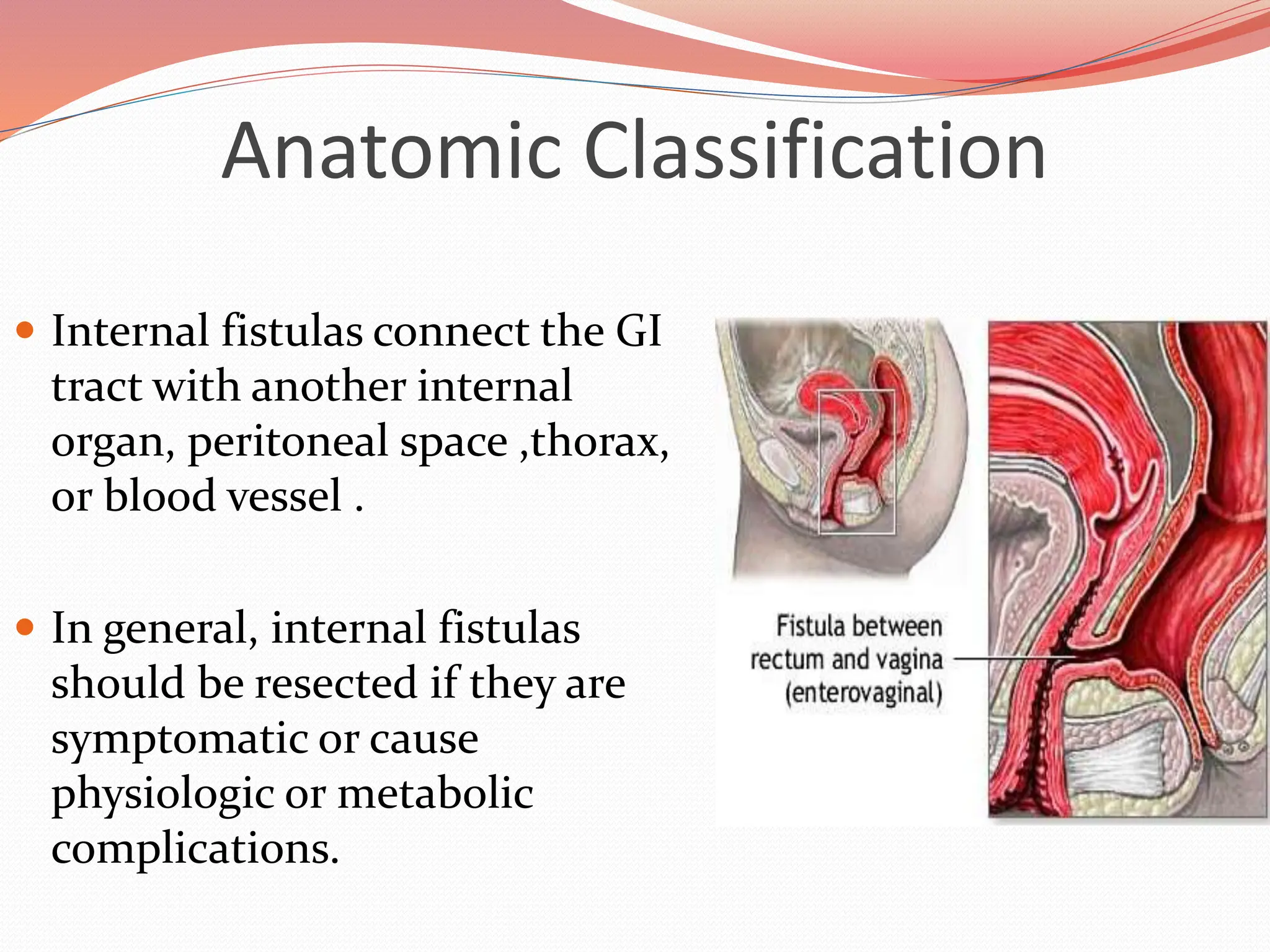
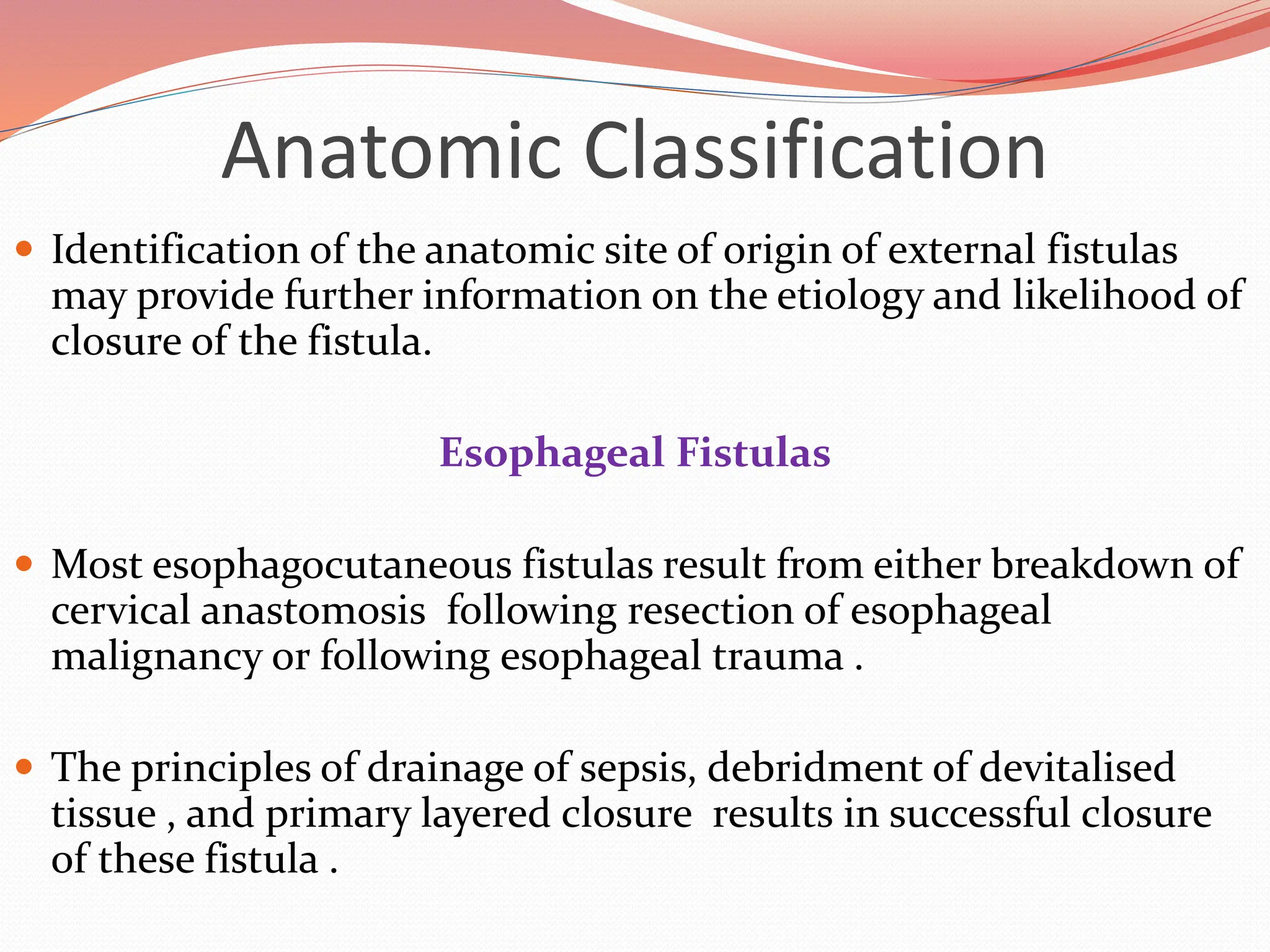
![Anatomic Classification
Gastric Fistulas
The most common cause gastrocutaneous fistula formation is the
removal of a gastrostomy feeding tube.
Nearly 90% of patients develop fistula when the tube had been in situ
for more than 9 months.
There are numerous reports of resolution of these fistulas with fibrin-
glue injection rather than operative closure.
The rate of gastrocutaneous fistula following operations for
nonmalignant processes such as ulcer disease, reflux disease, and obesity
is between 0.5% and 3.9%.
Gastrointest Endosc 2004;59:296 [PubMed: 14745411]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-12-2048.jpg)
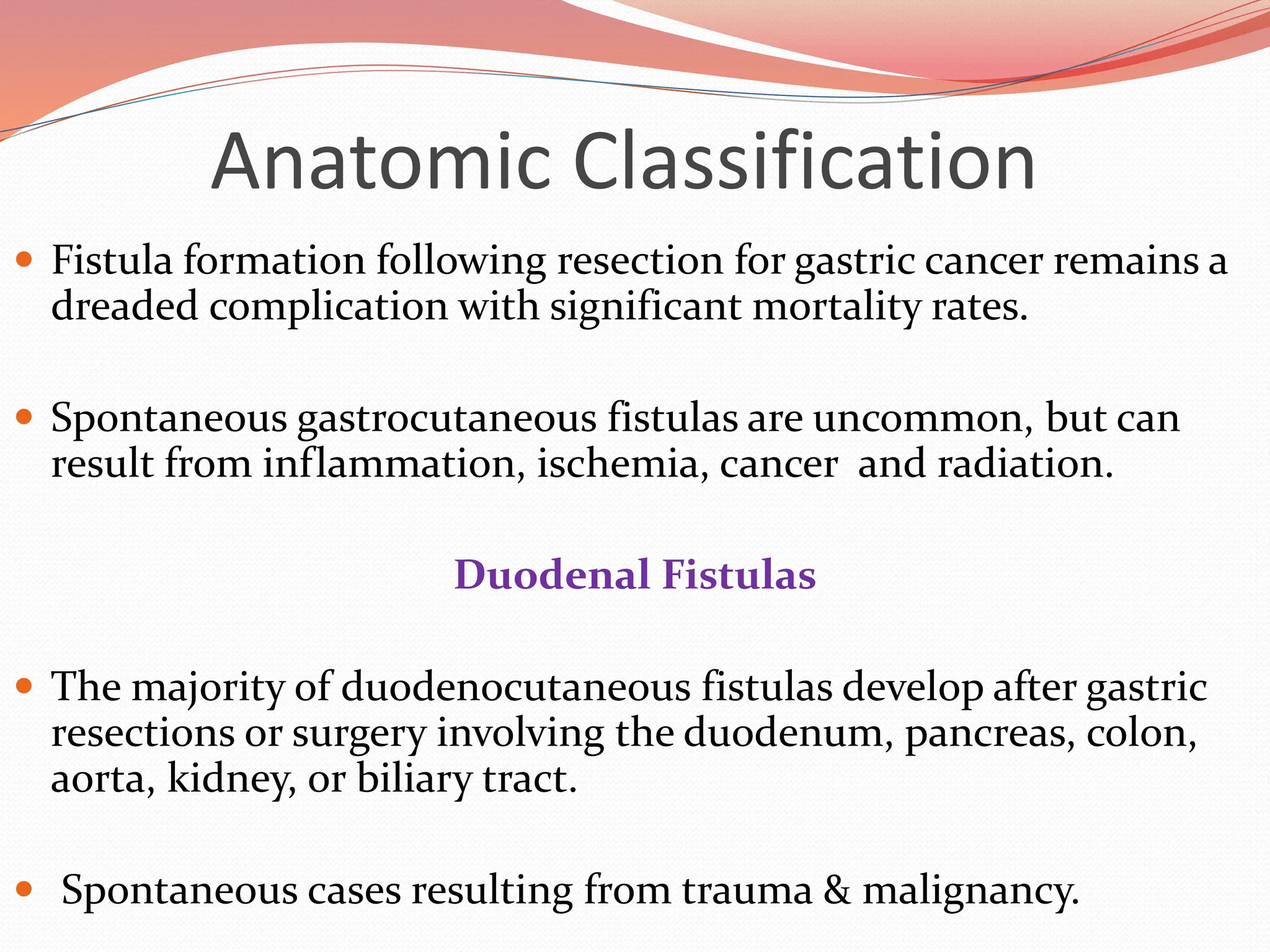
![Anatomic Classification
Edmunds and associates reported a decreased spontaneous
closure rate with lateral duodenal fistulas when compared to
duodenal stump fistulas.[1]
Small Bowel Fistulas
The majority of gastrointestinal cutaneous fistulas arise from the
small intestine.
Seventy to ninety percent of enterocutaneous fistulas occur in
postoperative period.
1.theAnn Surg 1960;152:445 [PubMed: 13725742]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-14-2048.jpg)


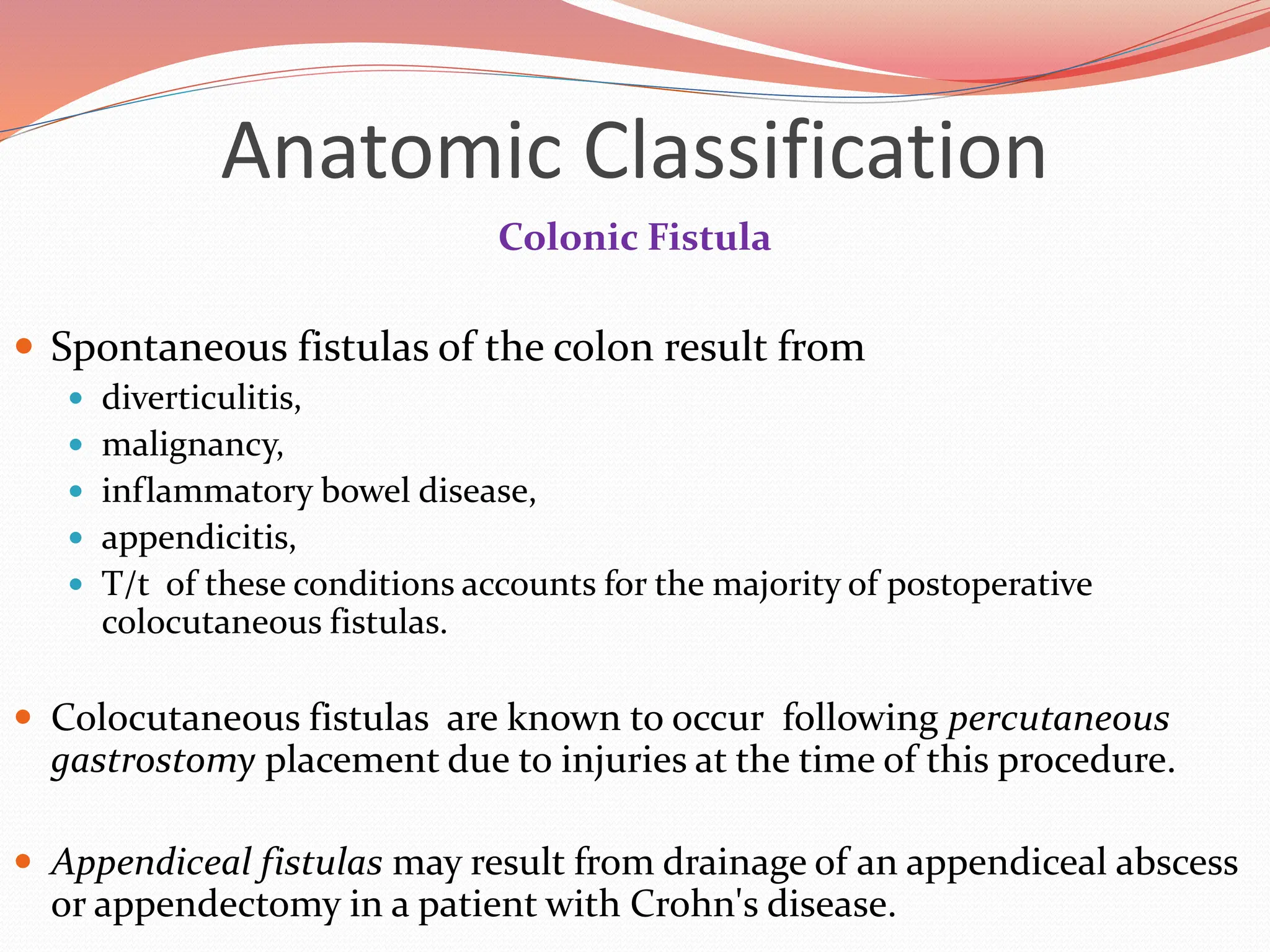

![Physiologic Classification
Enterocutaneous fistulas cause
the loss of fluid,
minerals,
trace elements,
and protein,
allow the release of irritating and caustic substances onto the skin.
Accurate measurement of both the amount and nature of enterocutaneous effluent
allows for accurate replacement.
Fistulas may be divided into 3 types :-
high-output (>500 mL per day),
moderate-output (200–500 mL/day),
low-output (<200 mL/day) groups.
Classification of enterocutaneous fistulas by the amount of daily output provides
information regarding mortality and in recent series may predict spontaneous
closure.[1,2]
1.British J Surg 1989;76:676 [PubMed: 2504436]
2.J Am Coll Surg 1999;188:483 [PubMed: 10235575]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-19-2048.jpg)
![Physiologic Classification
More recently, Levy and colleagues reported
50% mortality rate in patients with high-output fistulas
26% mortality with low-output fistulas
Campos and associates suggested that patients with low-output
fistulas were three times more likely to achieve closure without
operative intervention.
The reason for these different rates of closure is that,
high-output fistulas are likely to be of small-bowel origin,
low-output fistulas are likely to be of colonic origin [1].
1.Br J Surg 1989;76:676 [PubMed: 2504436]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-20-2048.jpg)



![Prevention
Mechanical and antibiotic bowel preparation reduce the amount
of particulate fecal material as well as colonic bacterial counts and
thus decrease the risk of fistula formation.
A recent meta-analysis of studies examining mechanical and
antibiotic bowel preparation suggests that bowel prep, may
increase the risk of anastomotic leakage, but confirmatory studies
are required before omission of preparation would be recommended
in practice.[1]
1.Slin K, Vicaut E, Panis Y et al. Meta-analysis of randomized clinical trials of colorectal surgery with or without
mechanical bowel preparation. Br J Surg 2004;91:1125](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-27-2048.jpg)





![Pathophysiology
The presence of infection adds to the stress on these patients and
limits the ability to achieve positive nitrogen balance.
Series of Hill and colleagues suggest that, until sepsis is
controlled, it is almost impossible to put patients into positive
nitrogen balance, even with any level of nutritional support.[1]
World J Surg 1988;12:191 [PubMed: 3134764]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-33-2048.jpg)





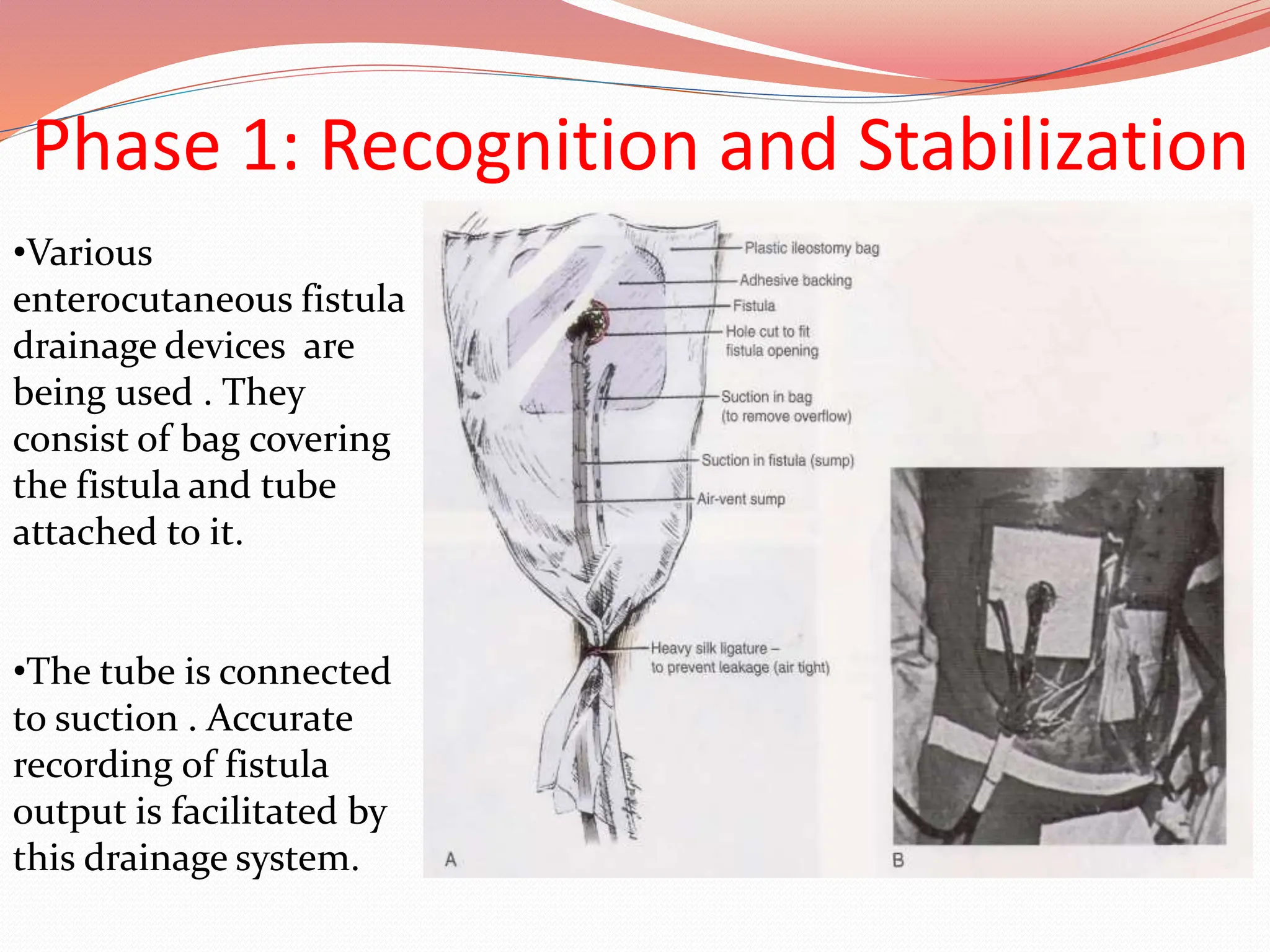
![Identification and Resuscitation
The diagnosis is now
clear [enterocutaneus
fistula ] and
management shifts
from routine
postoperative care to
the management of a
potentially critically ill
patient.](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-39-2048.jpg)










![Phase 1: Recognition and Stabilization
In a study of 100 patients with fistulizing Crohn's disease,
infliximab infusion resulted in complete response in 50 patients,
partial response in 22 patients, and no response in 28 patients.[1]
Whether this agent will be of use in patients without Crohn's
disease or ulcerative colitis remains to be determined
1.Am J Gastroenterol 2001;96:722 [PubMed: 11280541]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-51-2048.jpg)











![Phase 3: Decision
The timing of operative intervention for fistulas that are unlikely
to or fail to close is important.
Early operation is indicated to control sepsis not amenable to
percutaneous intervention.
First, 90–95% of fistulas that will spontaneous close typically do
so within 5 weeks of the original operation.
Furthermore, operation during the first 10 days to 6 weeks from
diagnosis of postoperative fistulas is made more difficult by the
"obliterative peritonitis" described by Fazio and associates.[1]
1.World J Surg 1983;7:481 [PubMed: 6624123]](https://image.slidesharecdn.com/enterocutaneousfistulla28-240222172004-b22a3bf0/75/Enterocutaneous-Fistula-general-surg-pptx-63-2048.jpg)












