The document provides an overview of the subject 'Basic Chemistry' for first-year computer technology students at Sanjivani K.B.P. Polytechnic, detailing the classification of polymers based on various criteria such as occurrence, monomer nature, structure, and thermal characteristics. It explains types of polymerization—addition and condensation—along with examples of thermoplastics and thermosetting polymers. Additionally, it lists applications of specific polymers, highlighting their uses in diverse fields like insulation, safety gear, and adhesives.



![Applications of polymer
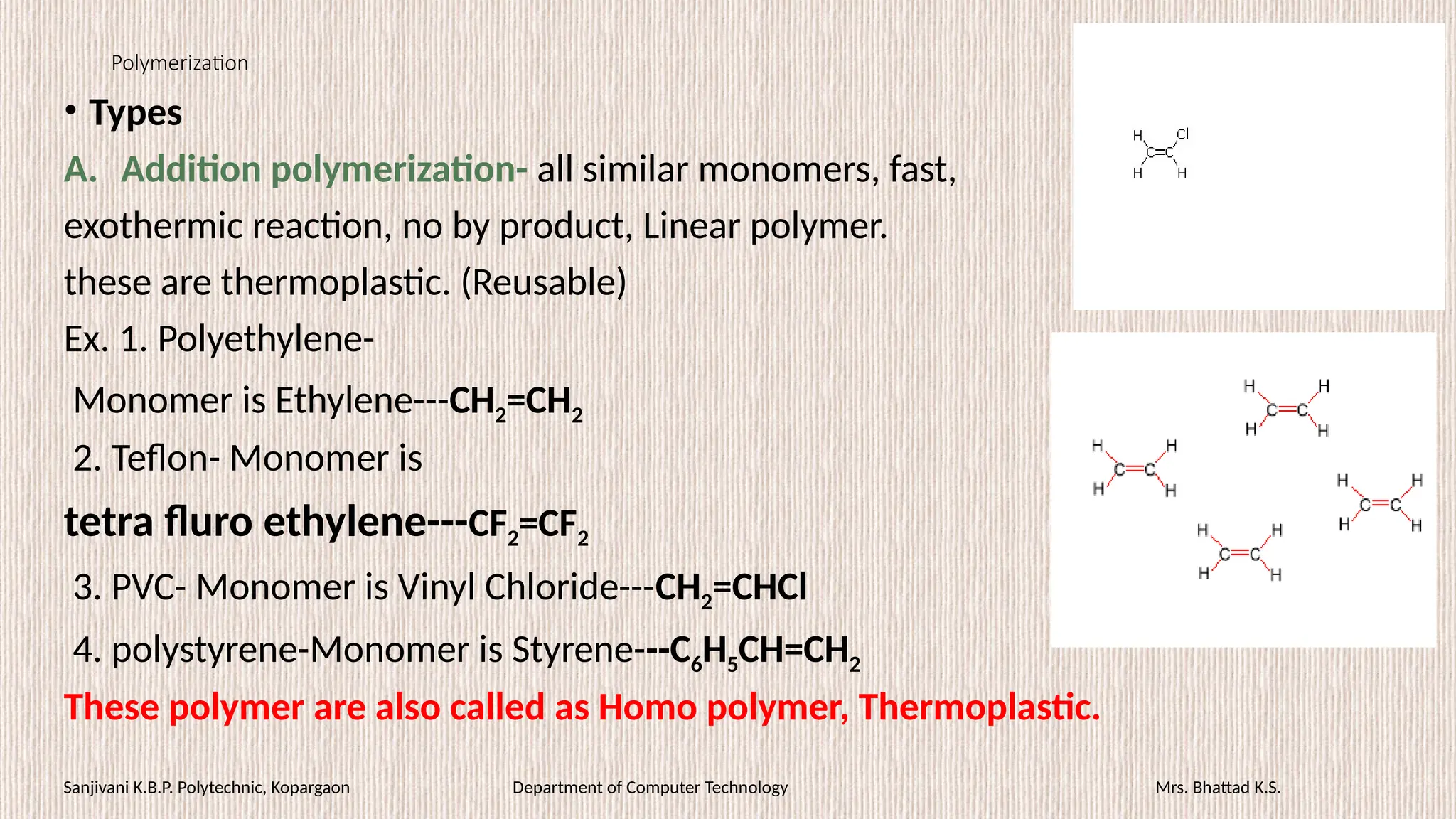
• PE- Insulation, wrapping, caps, toys, domestic appliances.
• PVC- tank lining, safety helmets, refrigerator components, tyres,
mudguards.
• PTFE- gaskets, packing, chemical carrying pipes, tubing, tanks, non
stick cookware etc.
• PS- Toys, combs, buckles, buttons, fancy article.
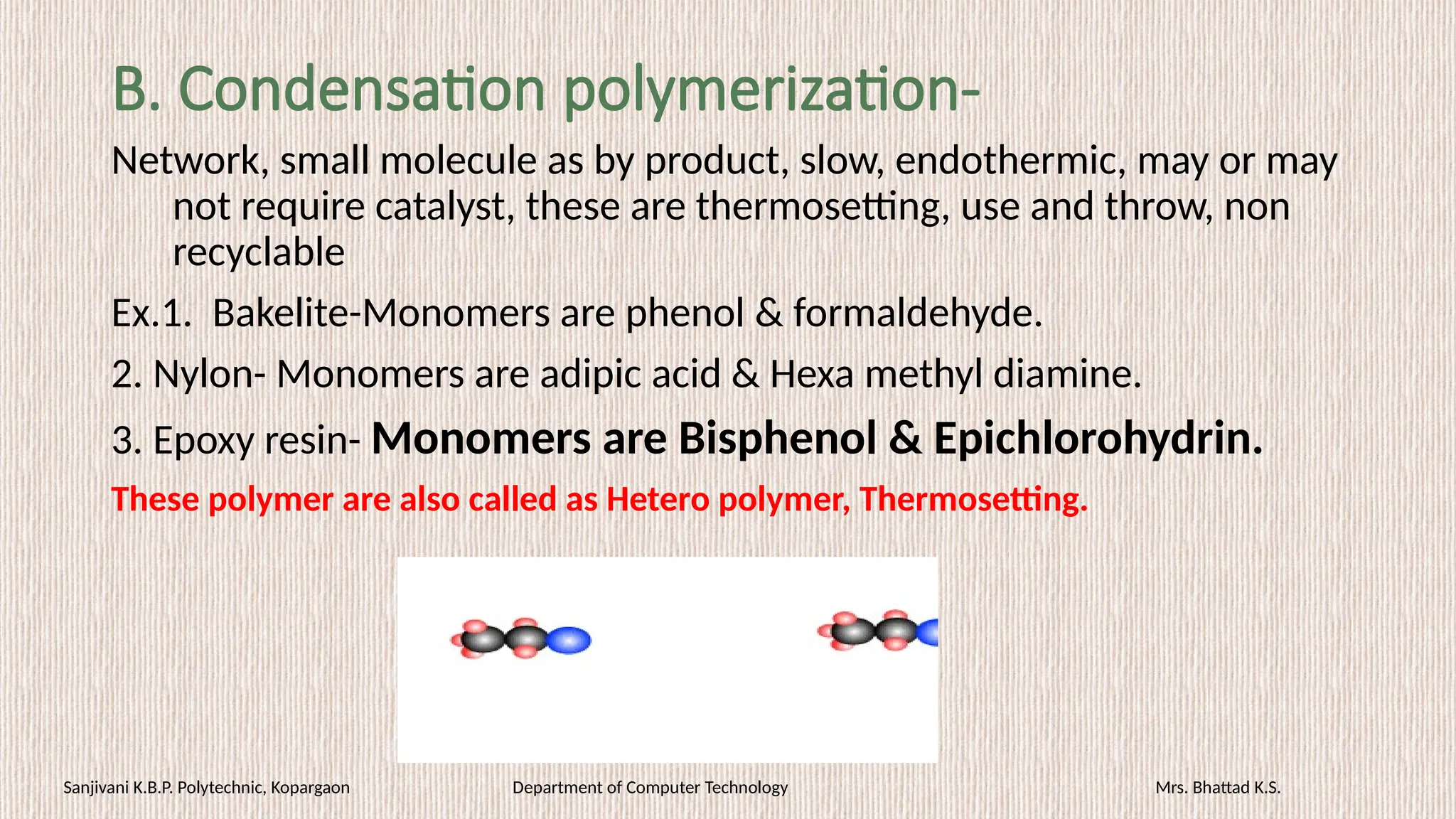
• PFR [Bakelite or phenoplast]- Switches, plugs, cabinet for TV, radio,
good adhesives.
• Epoxy resin- famous adhesives (Araldite), electrical bushings, etc.
Sanjivani K.B.P. Polytechnic, Kopargaon Department of Computer Technology Mrs. Bhattad K.S.](https://image.slidesharecdn.com/polymer-241119114459-e59559f2/75/Engineering-material-Polymers-pptx-7-2048.jpg)