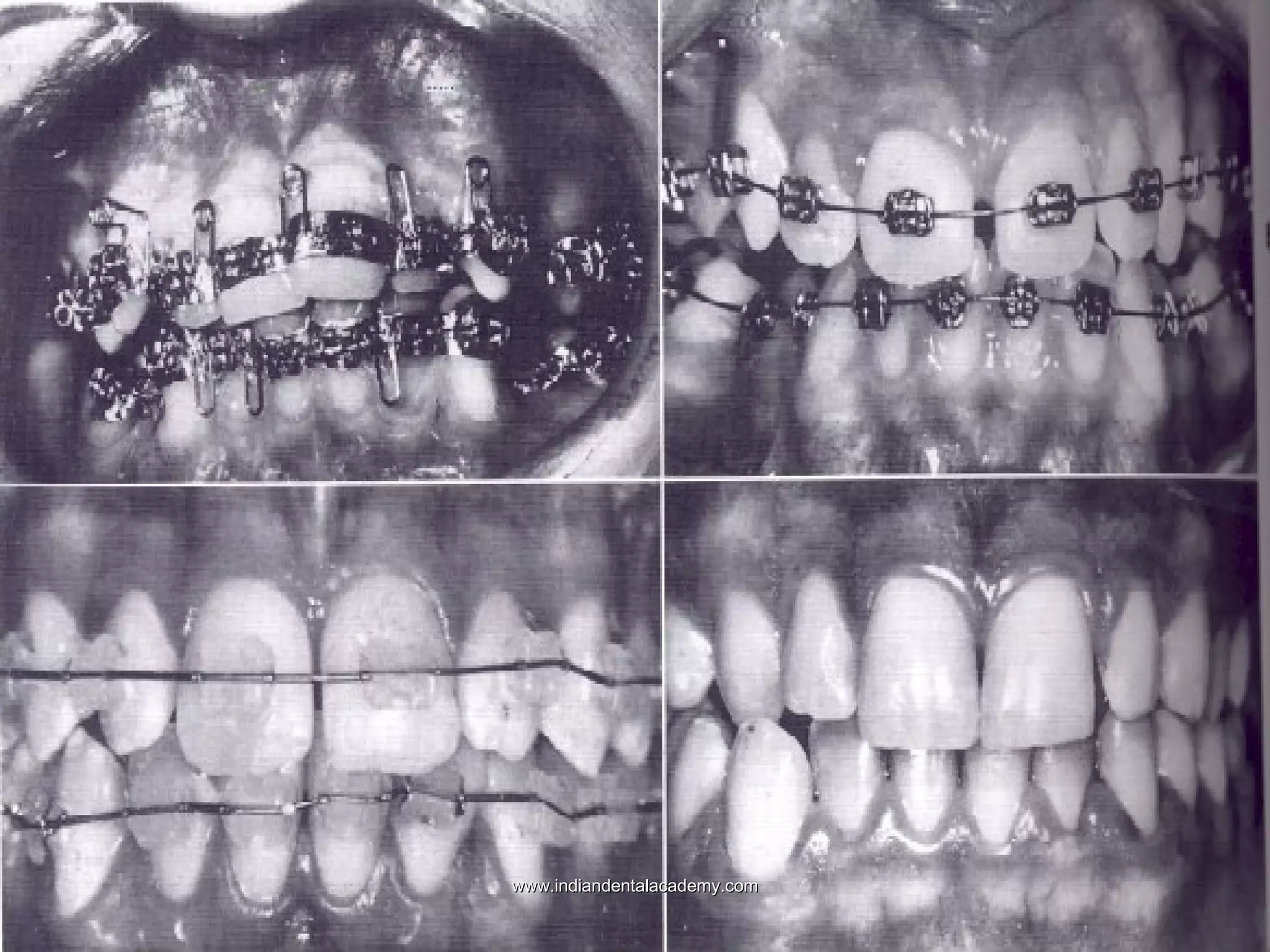
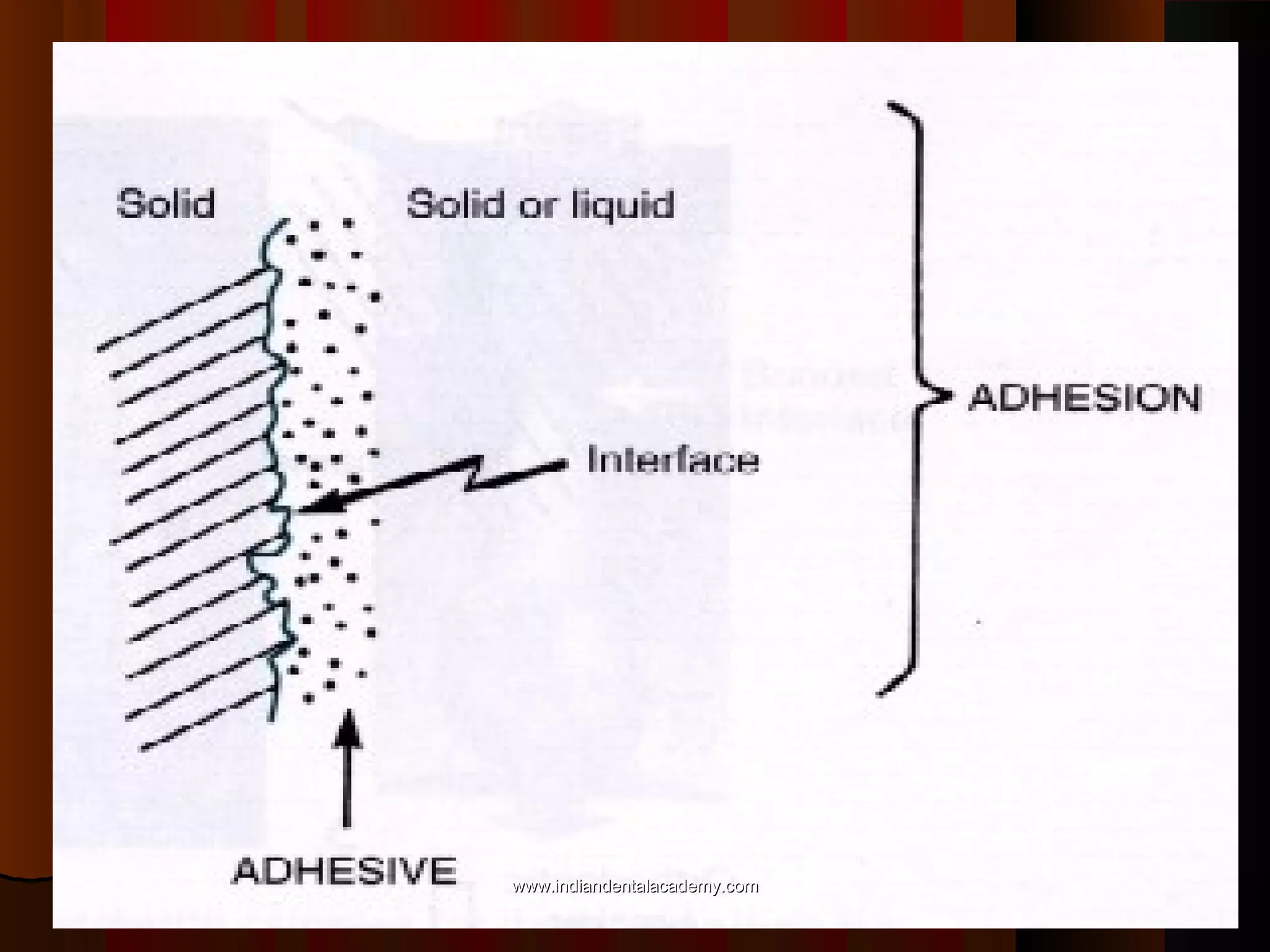
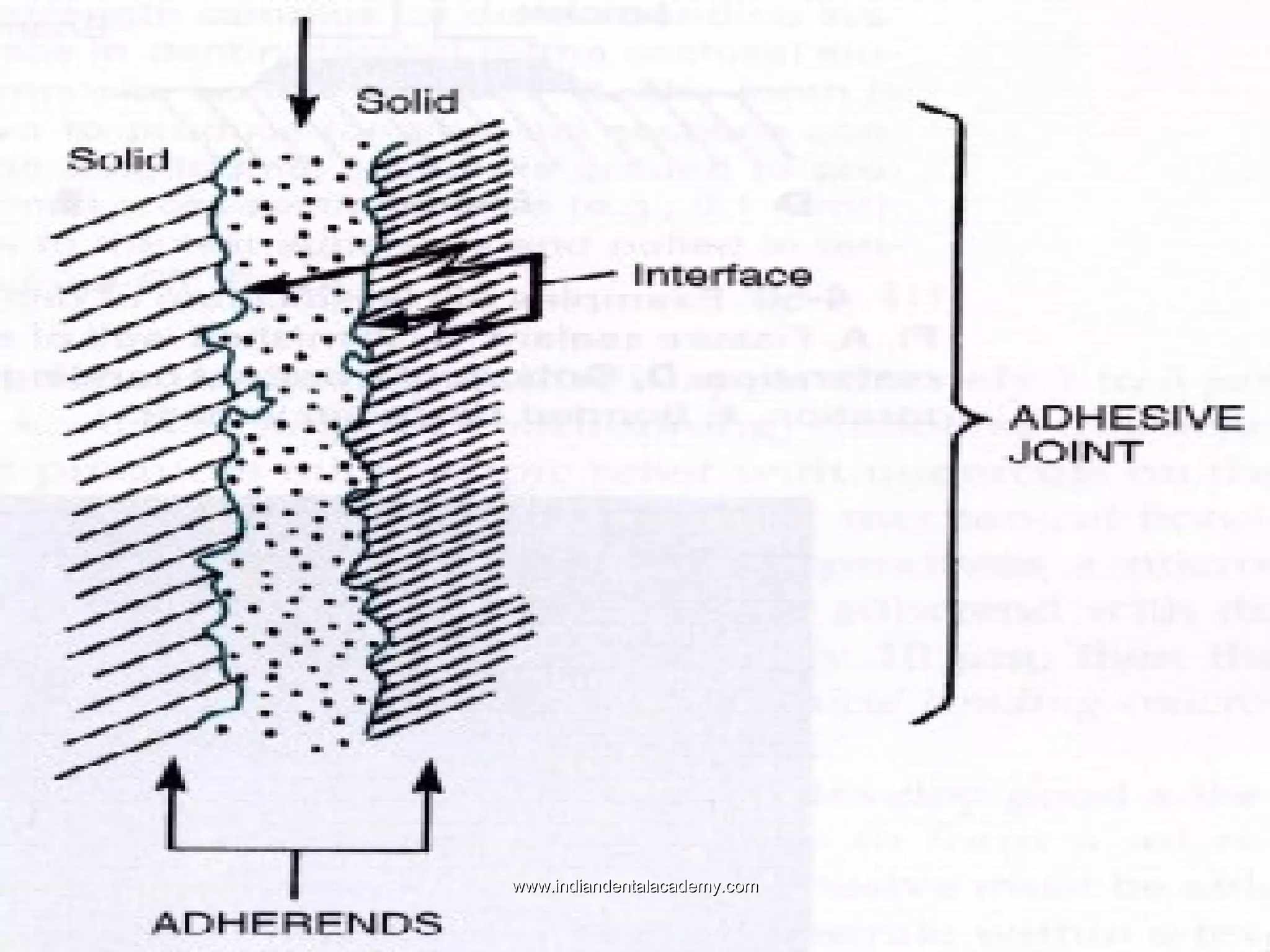
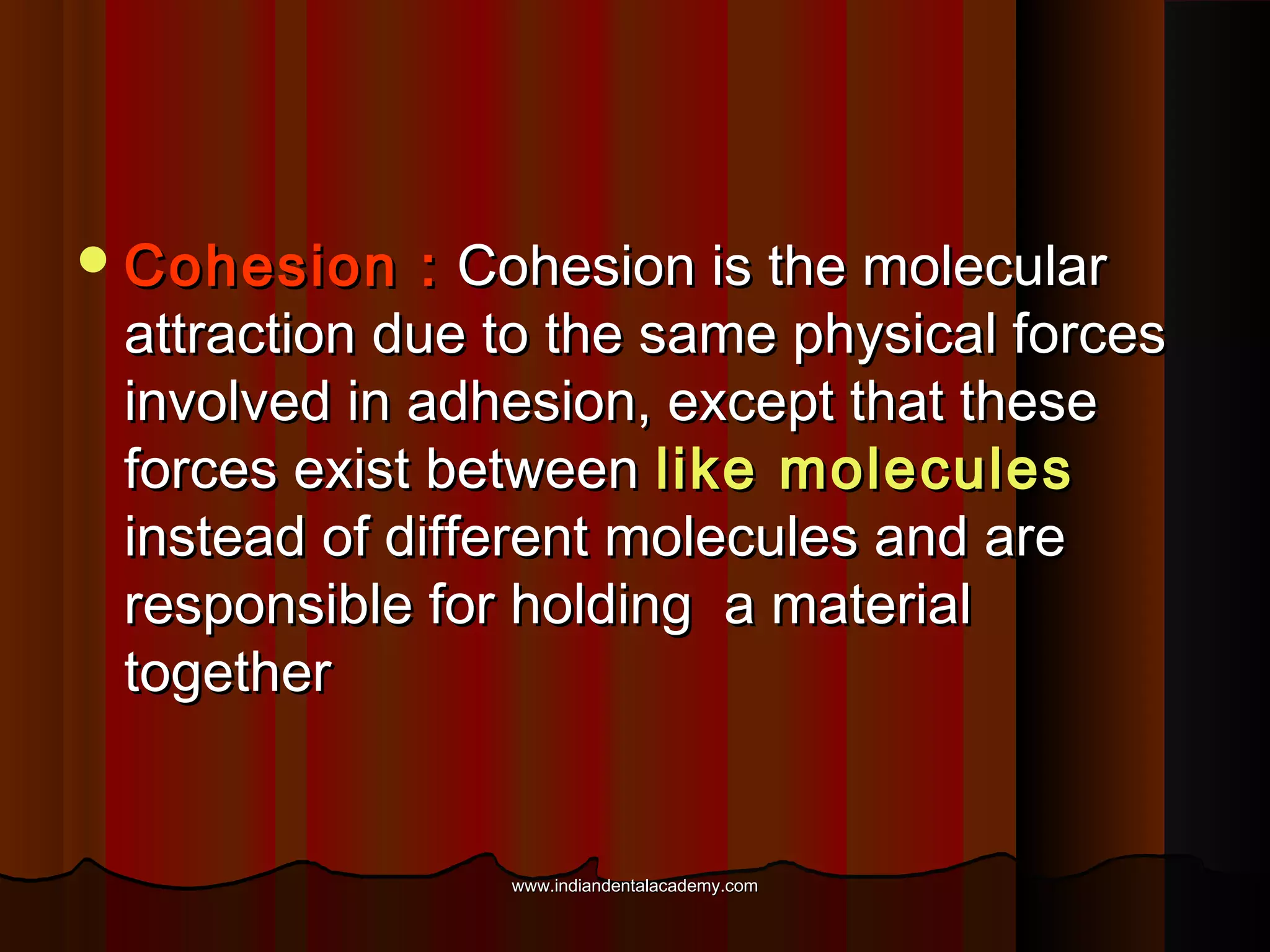
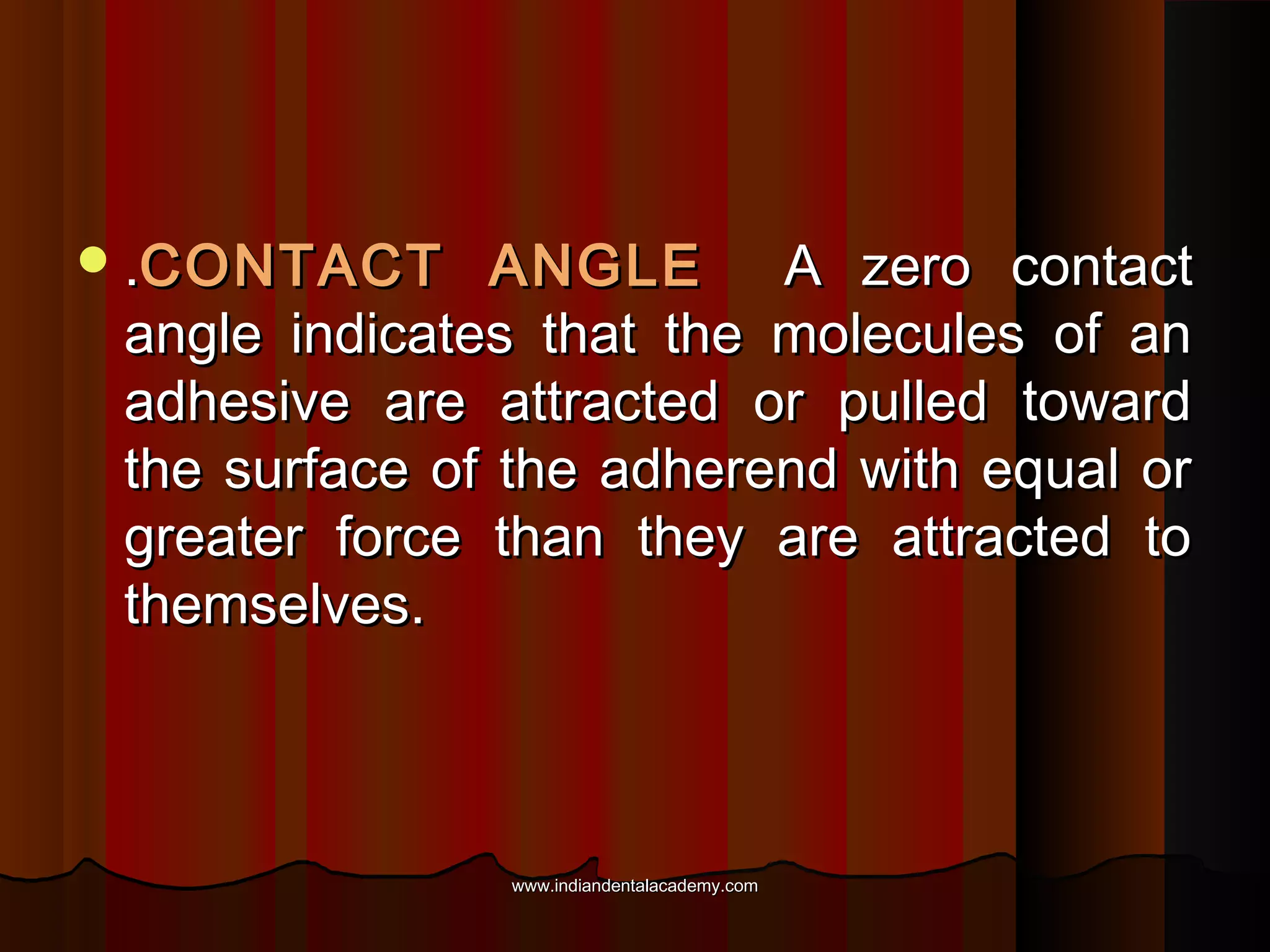
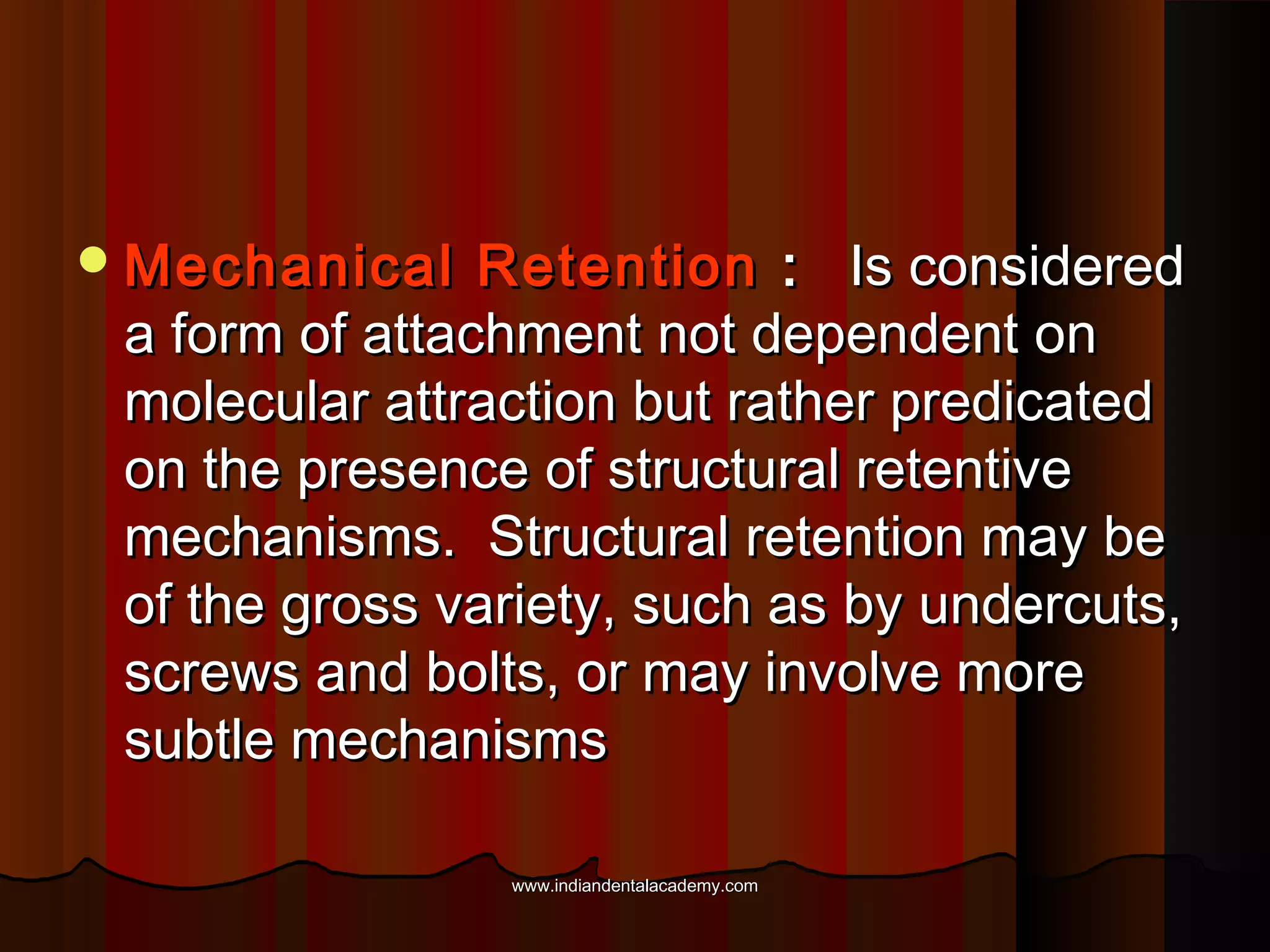
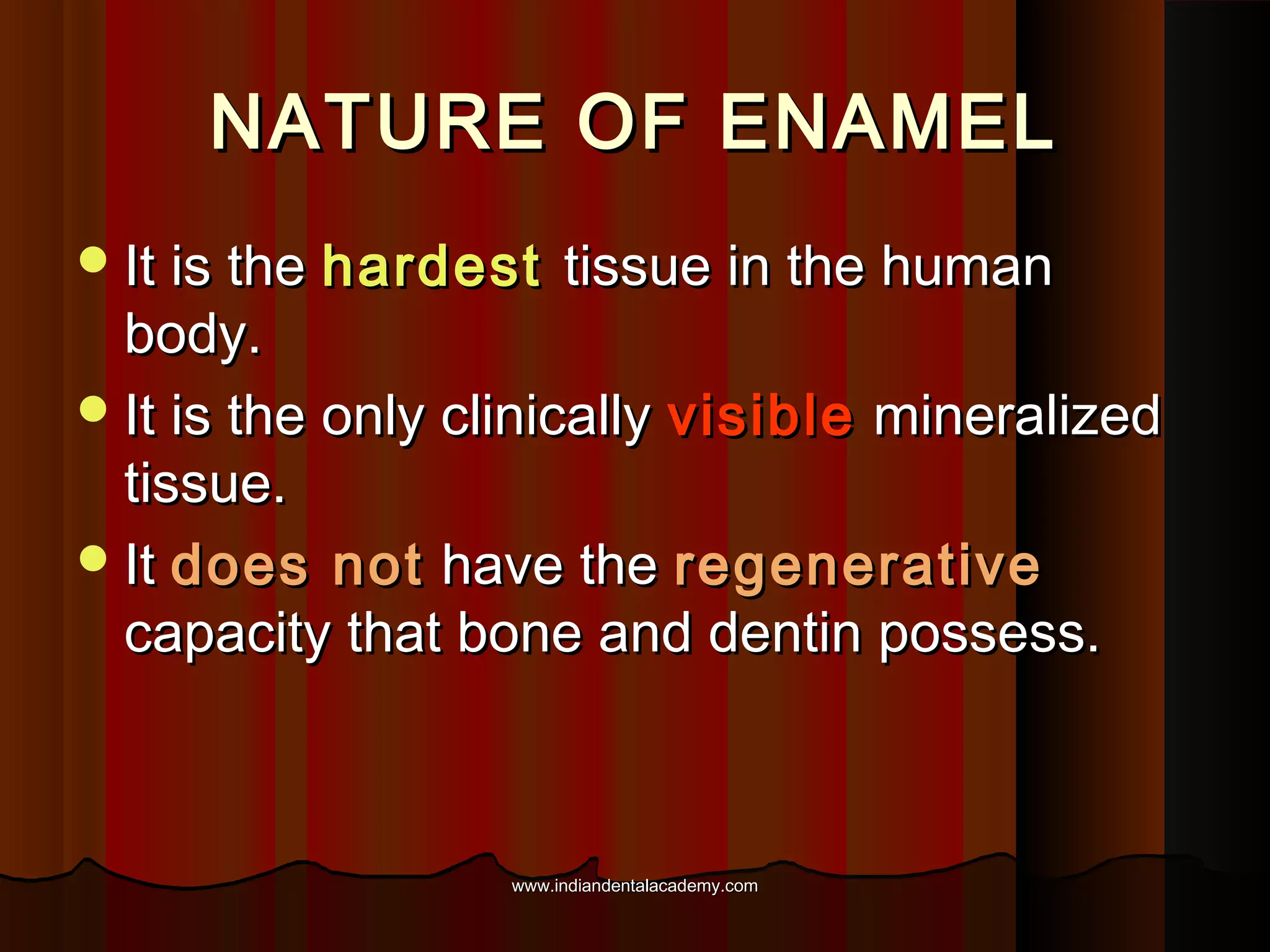
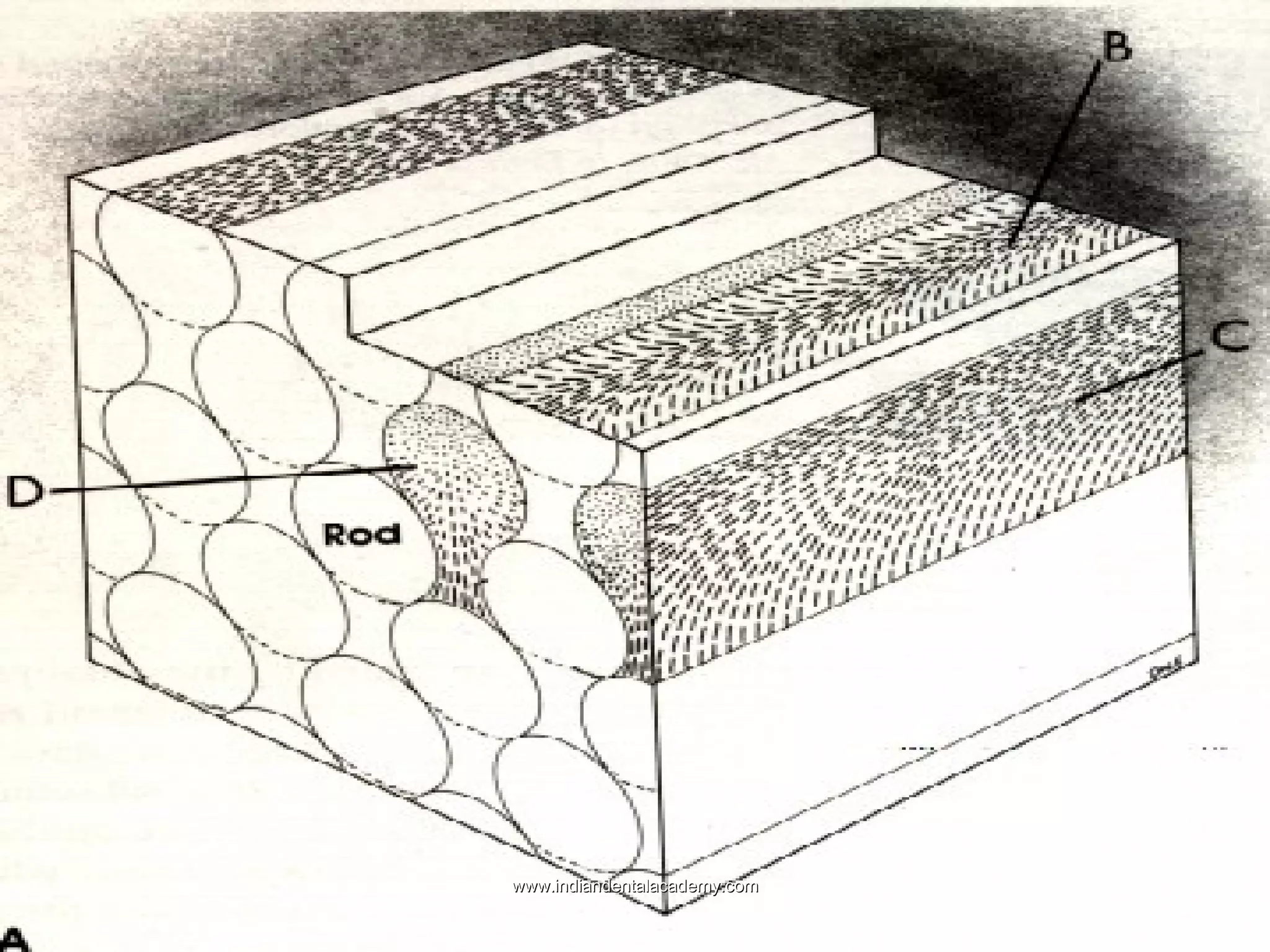
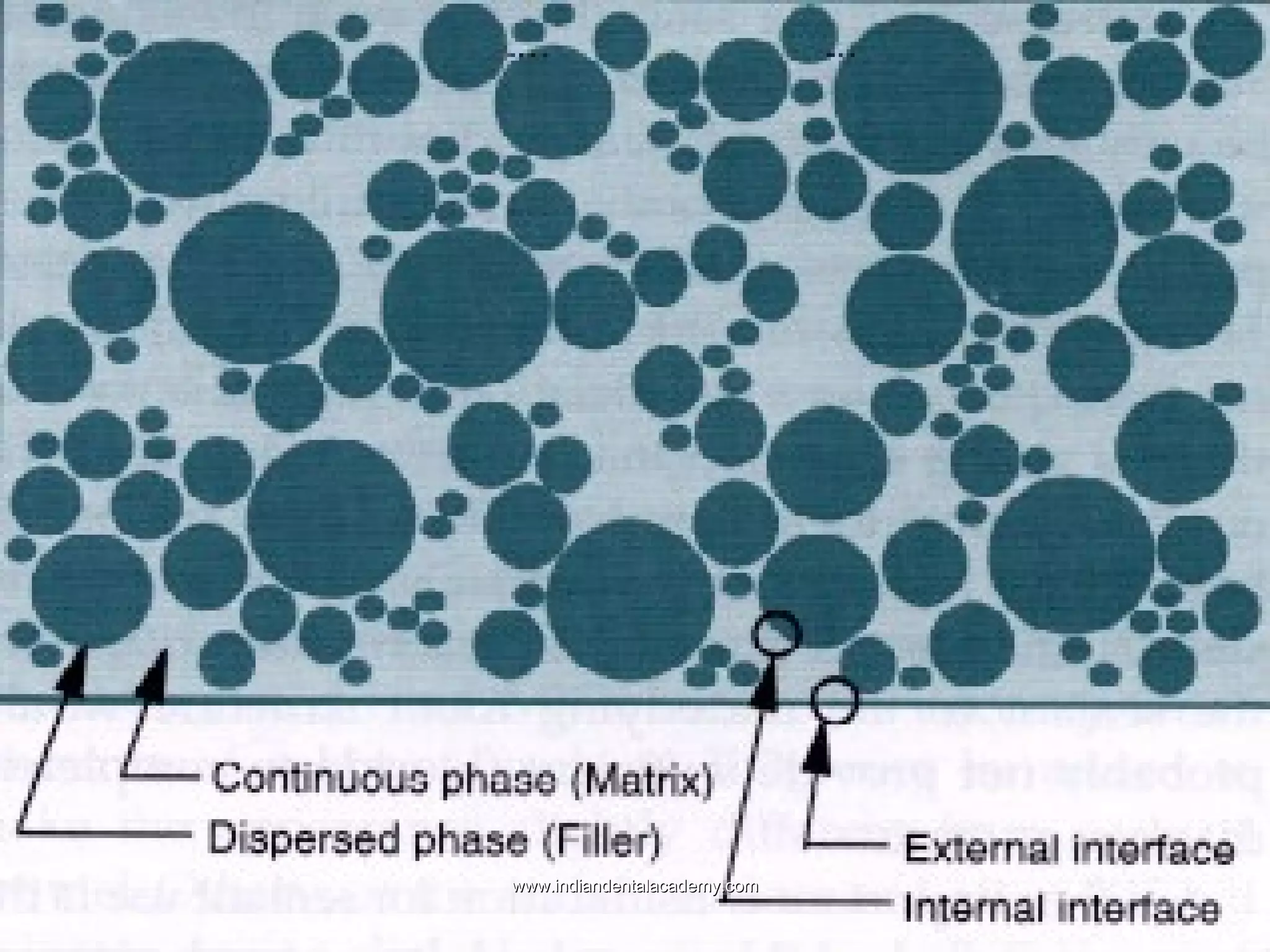
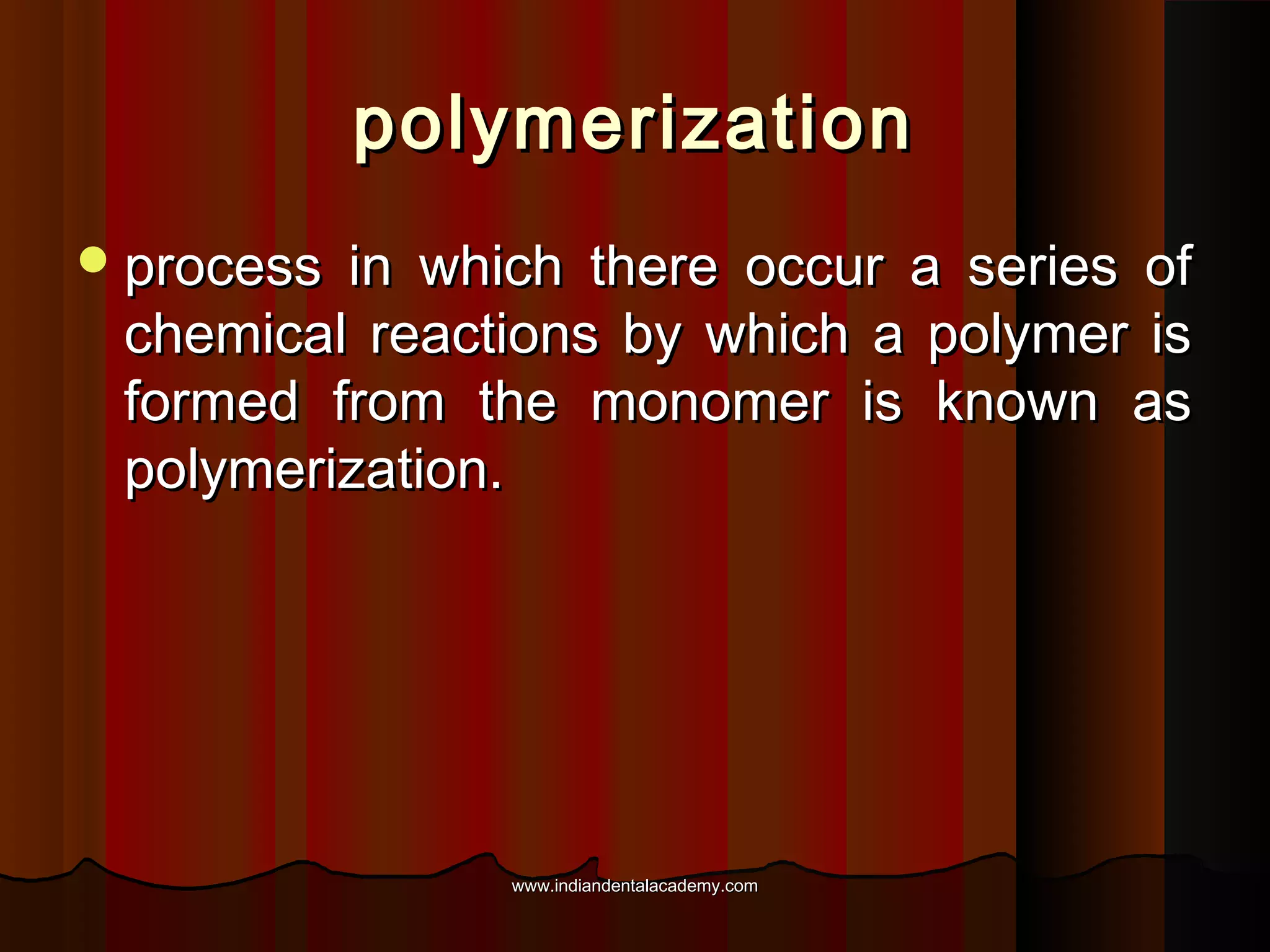
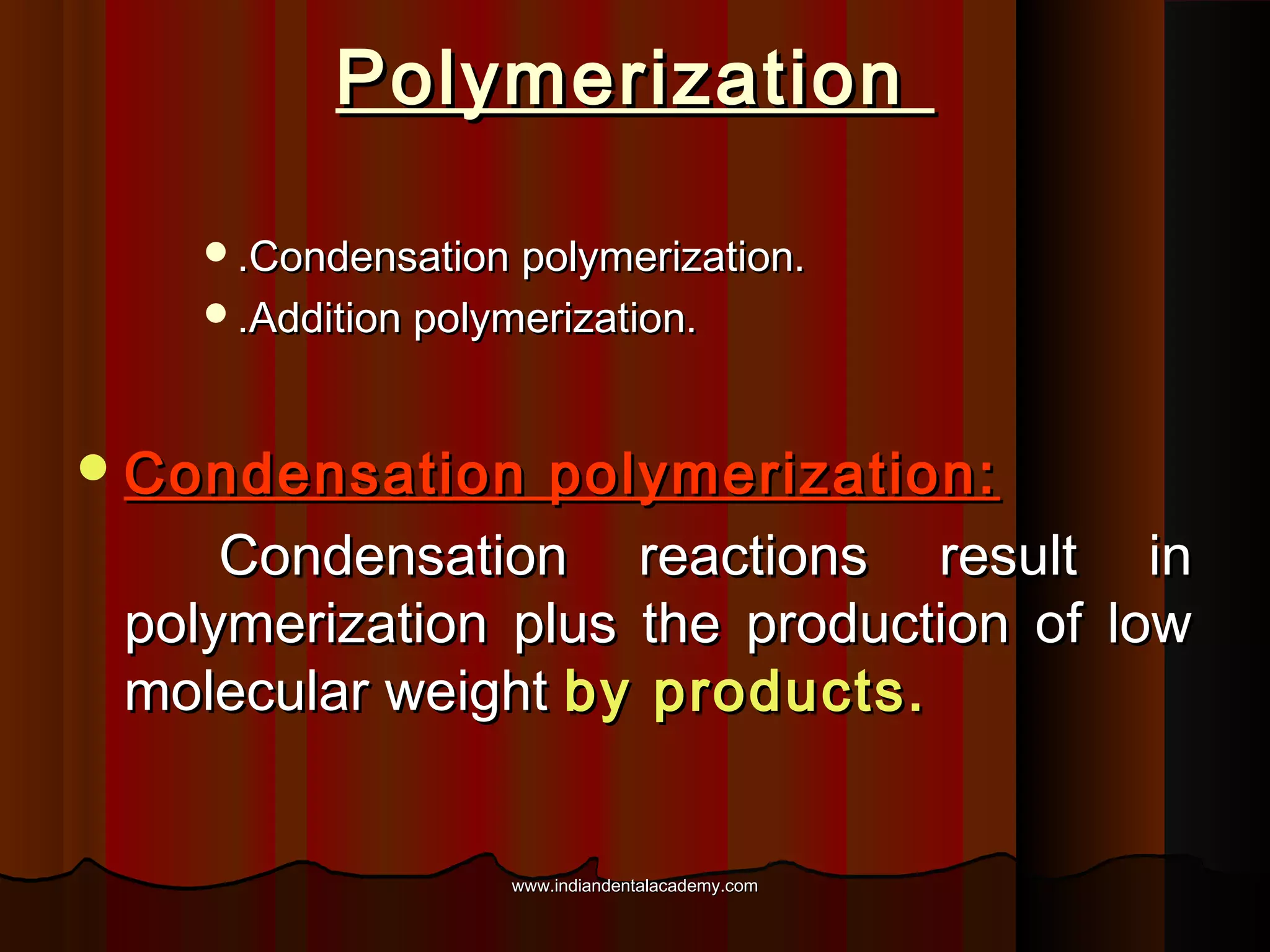
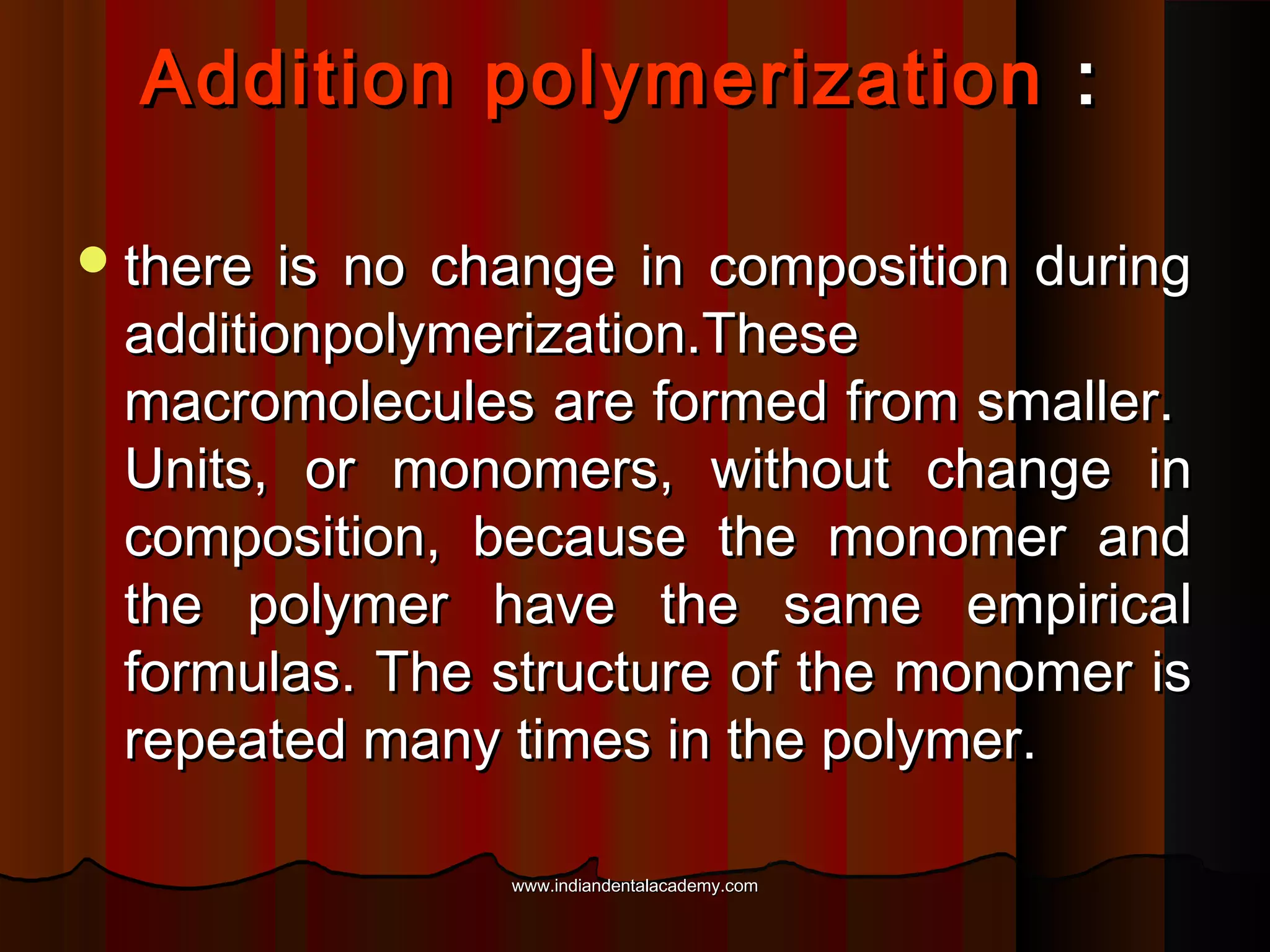
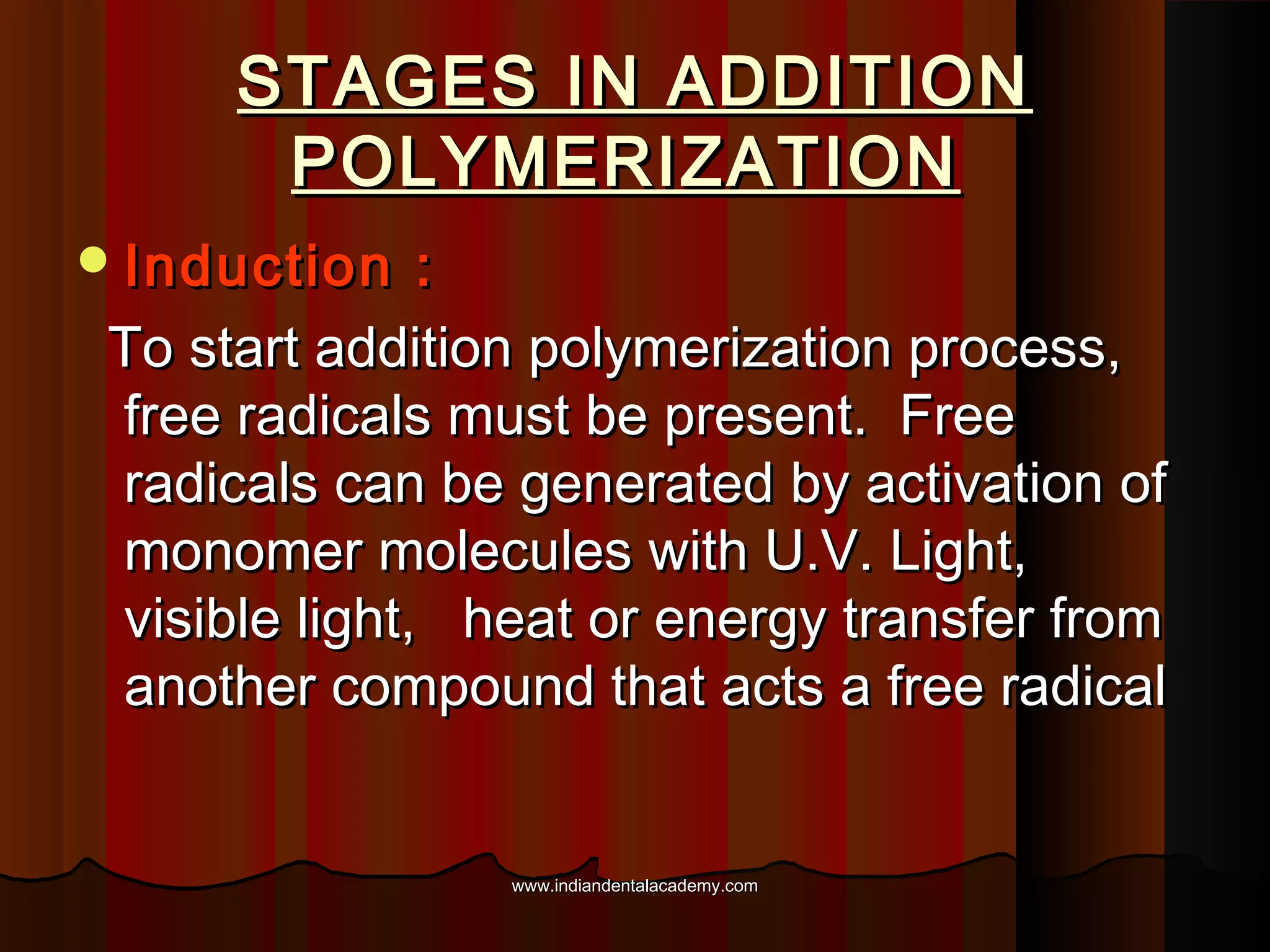
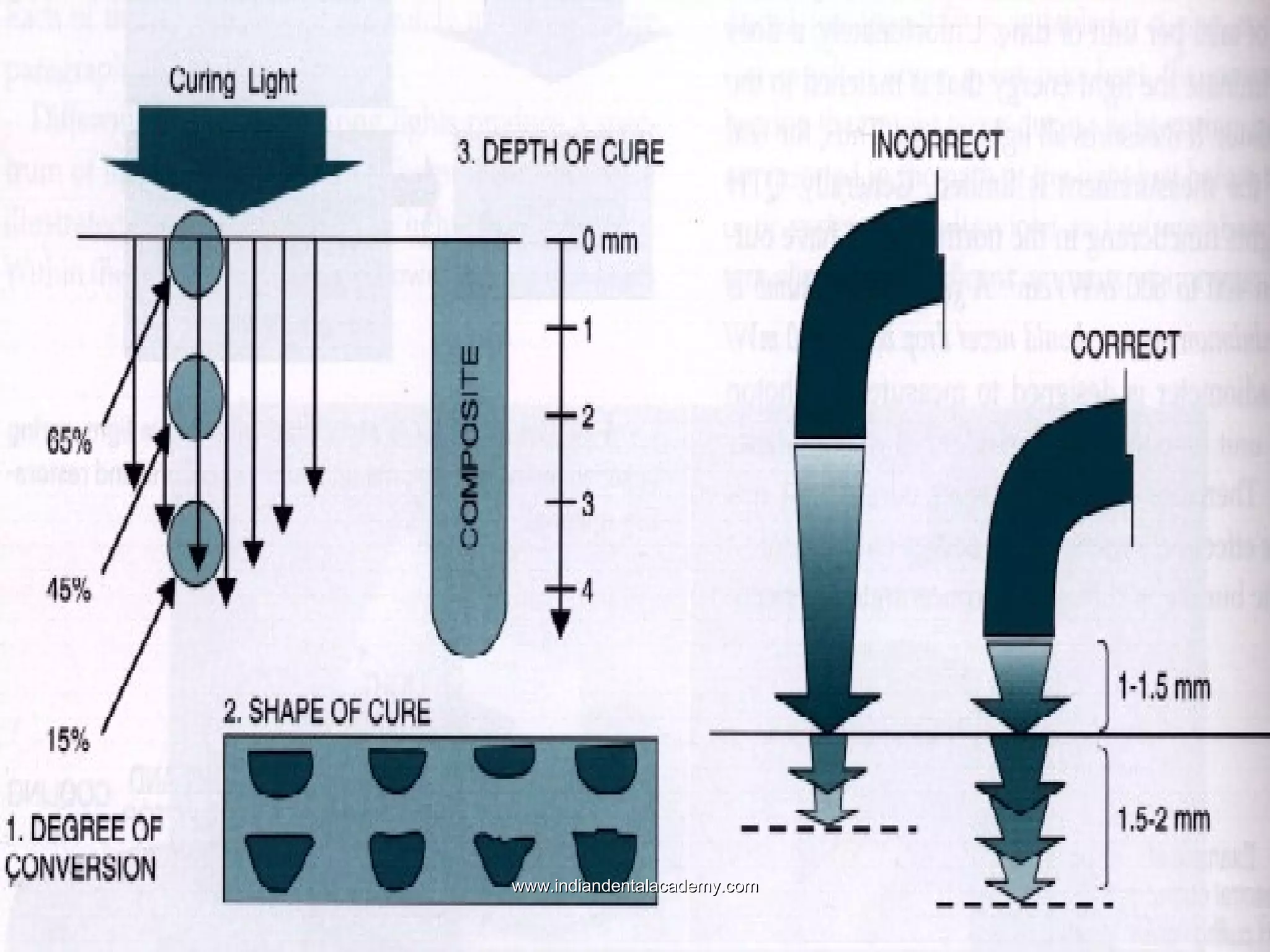
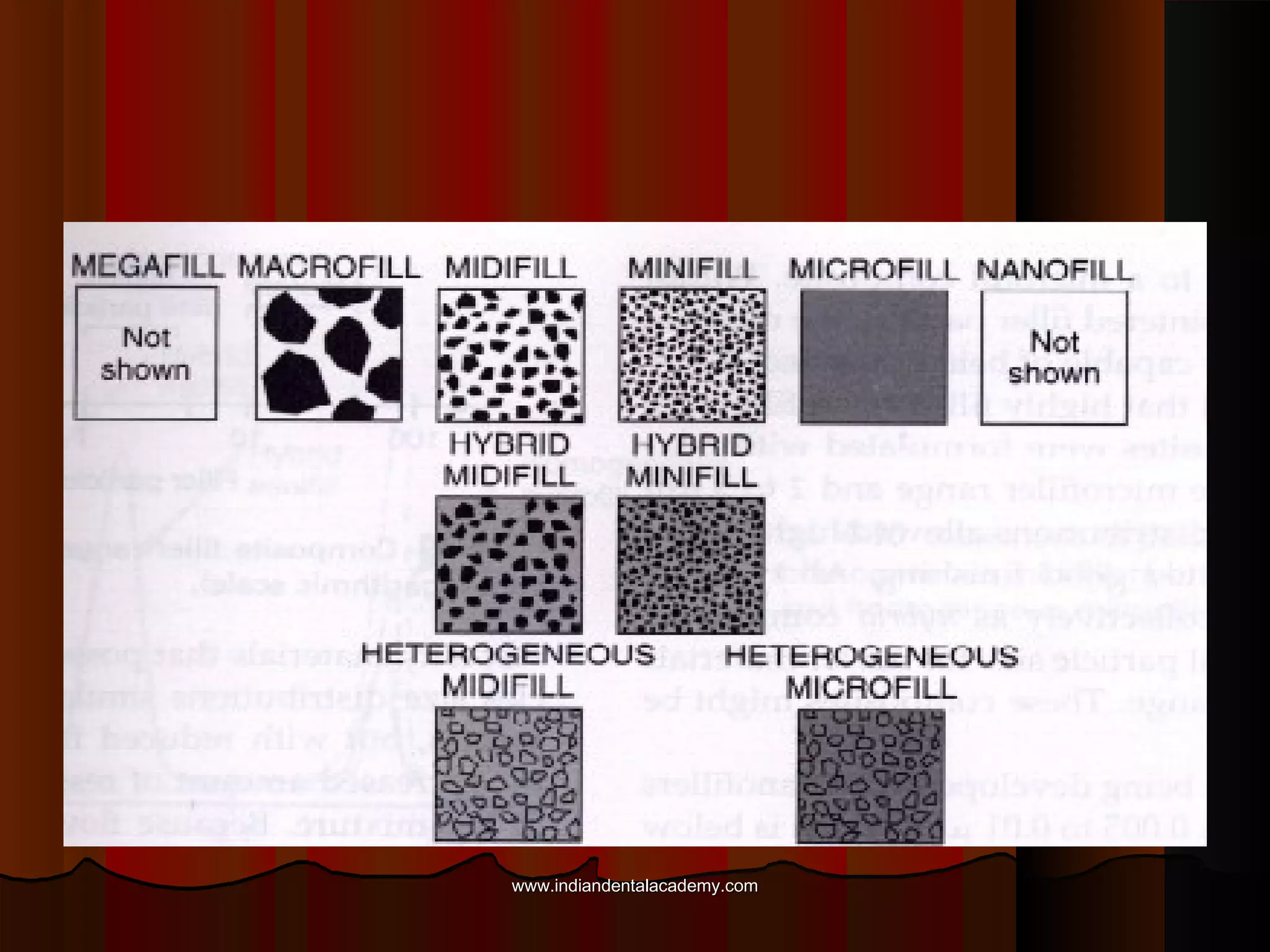
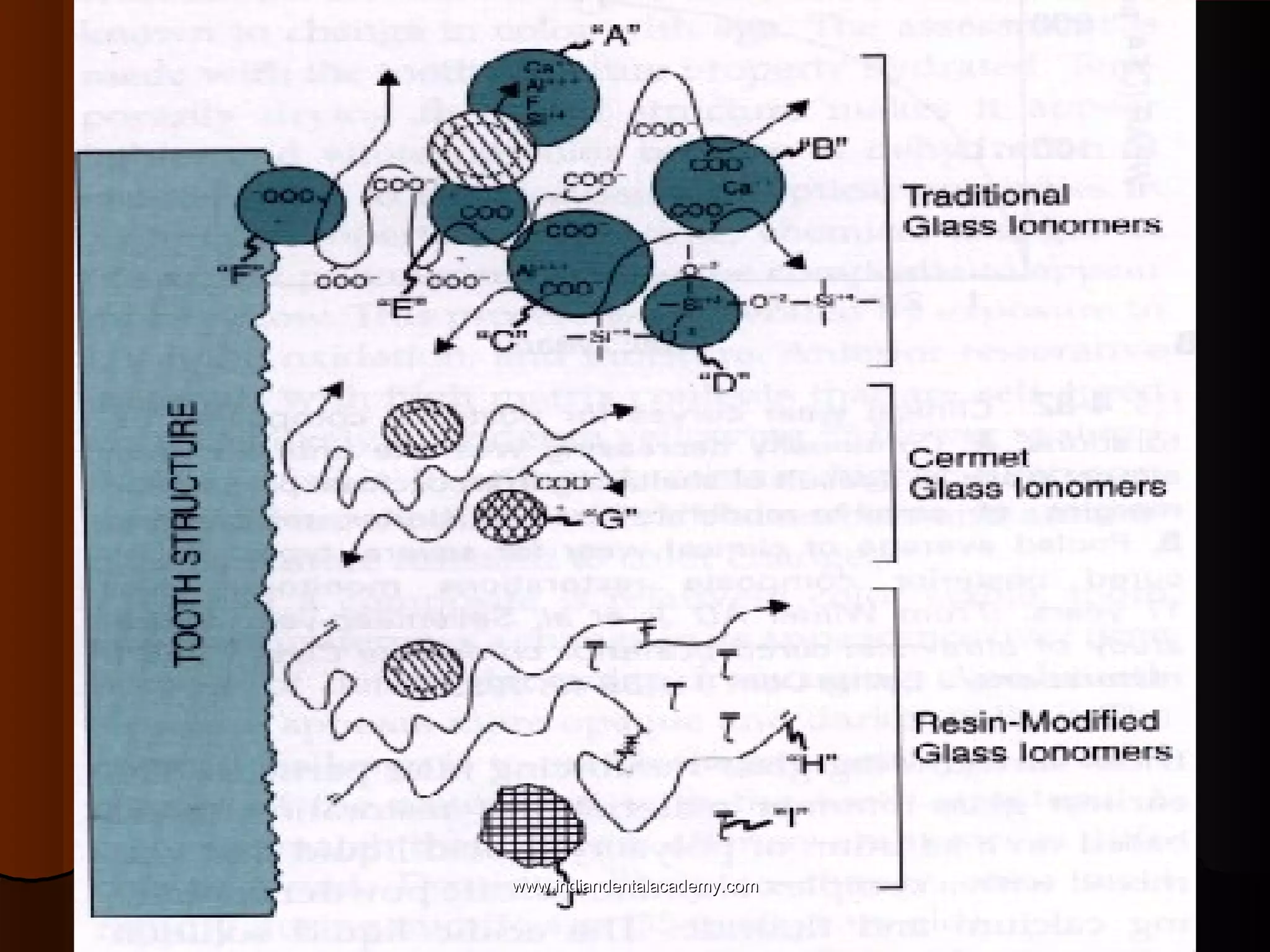
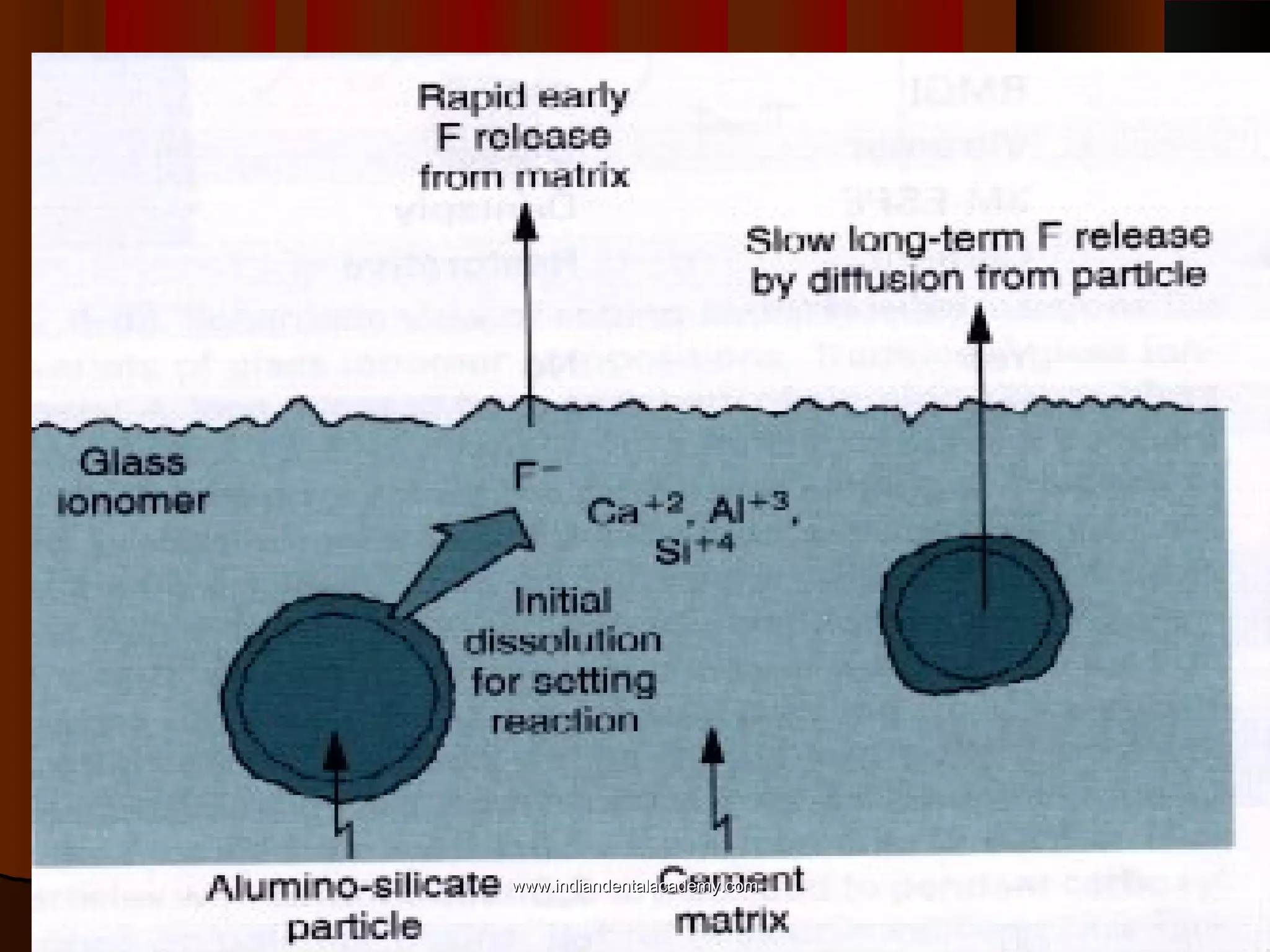
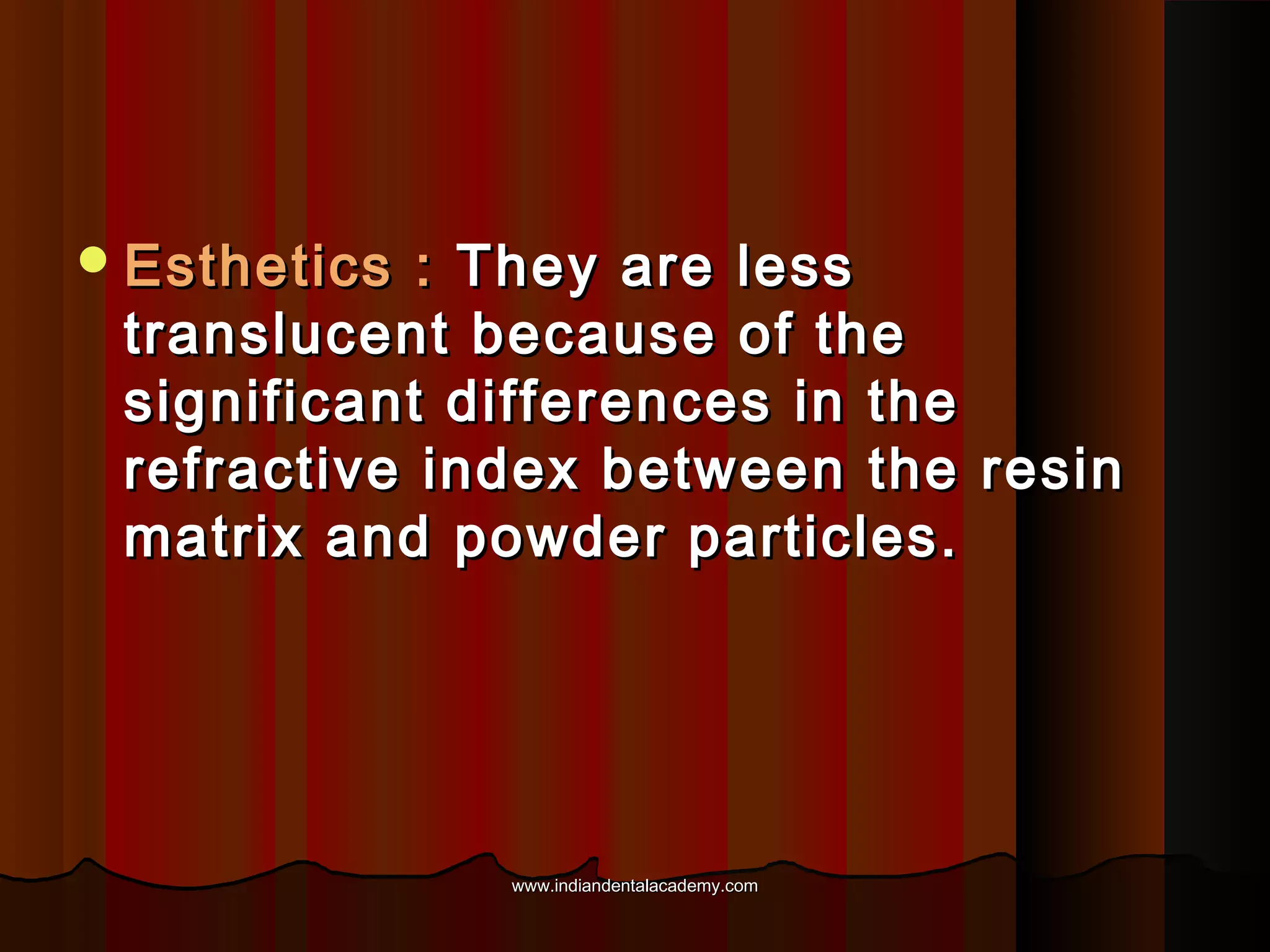
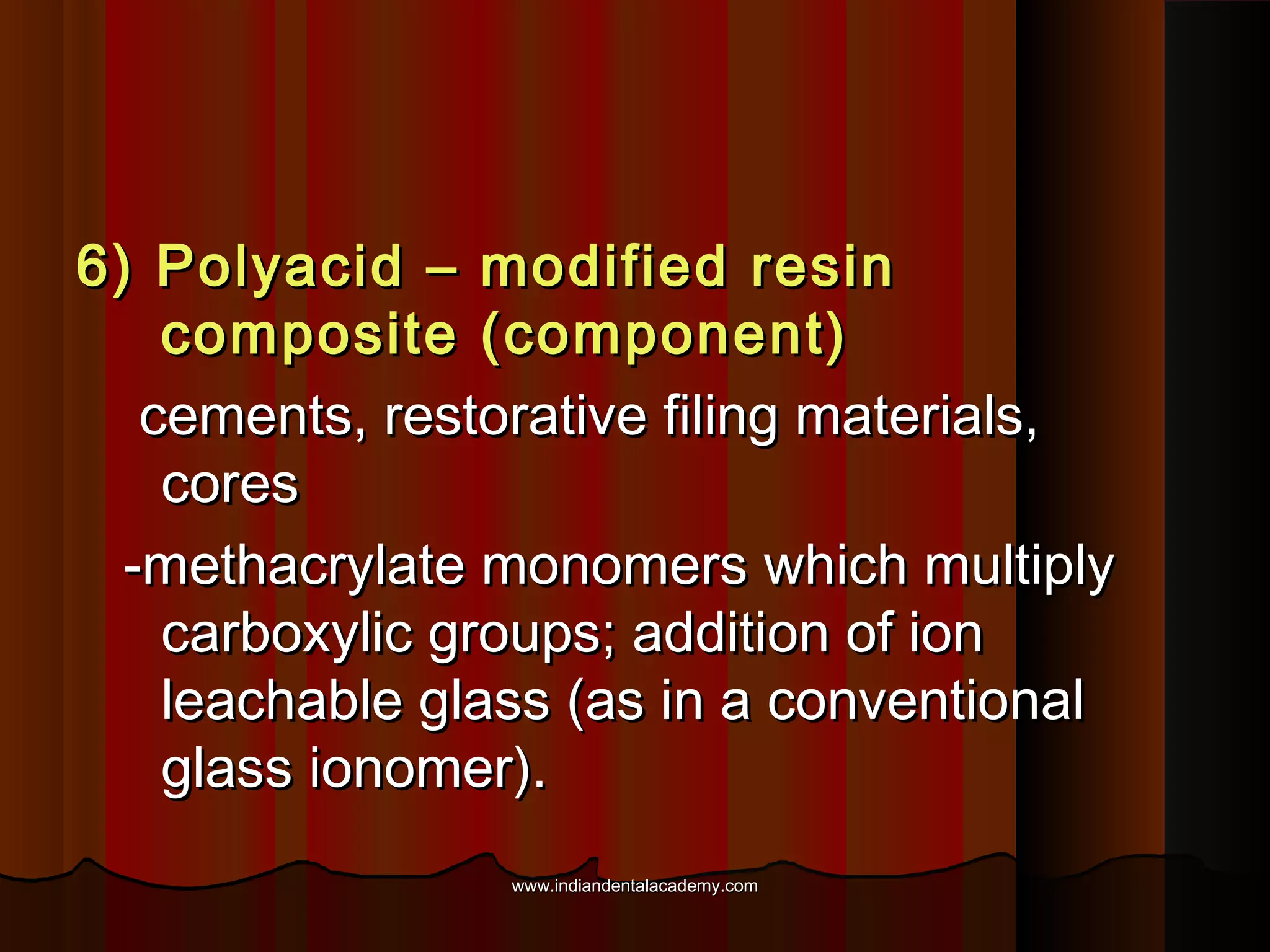
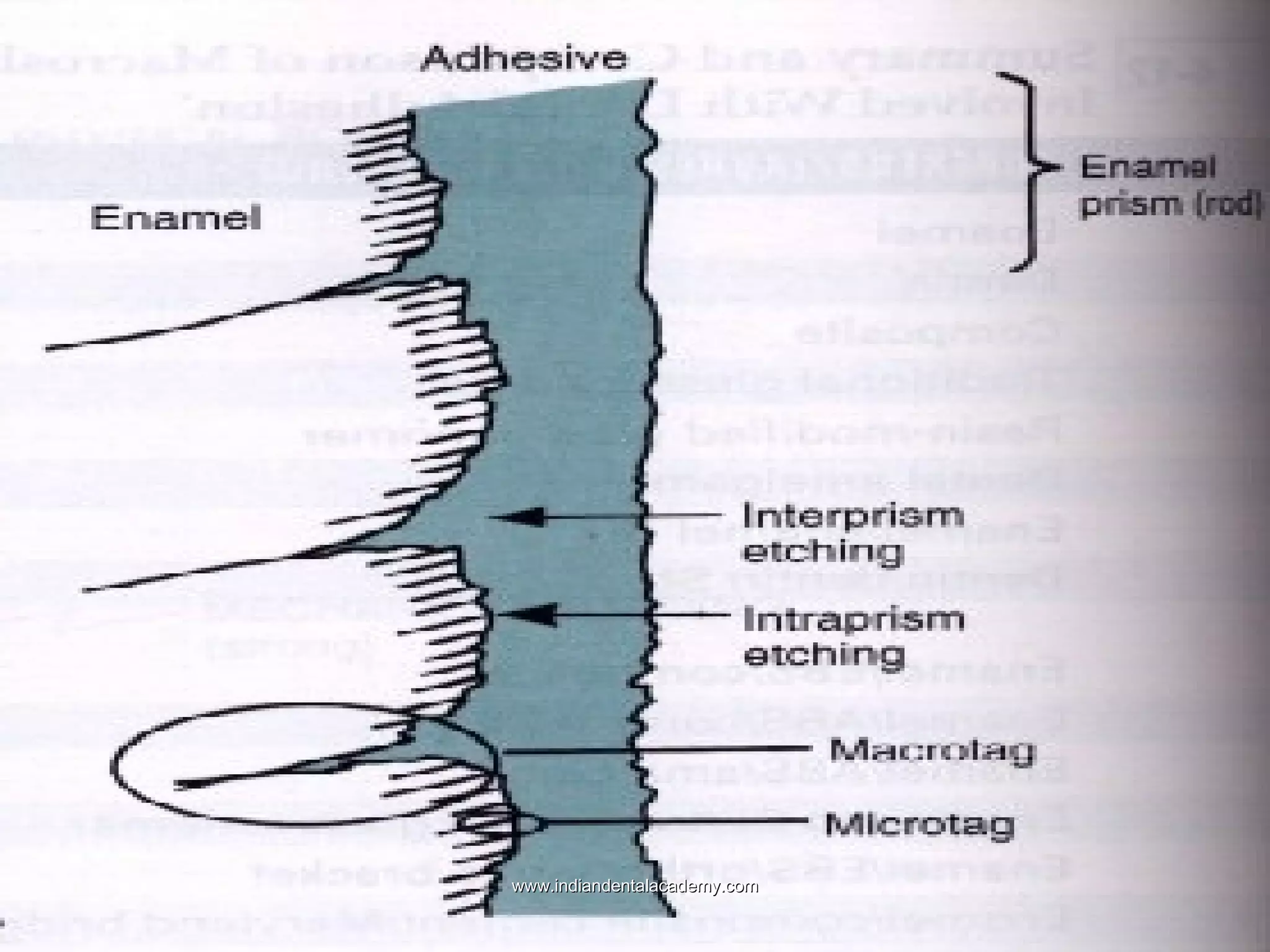
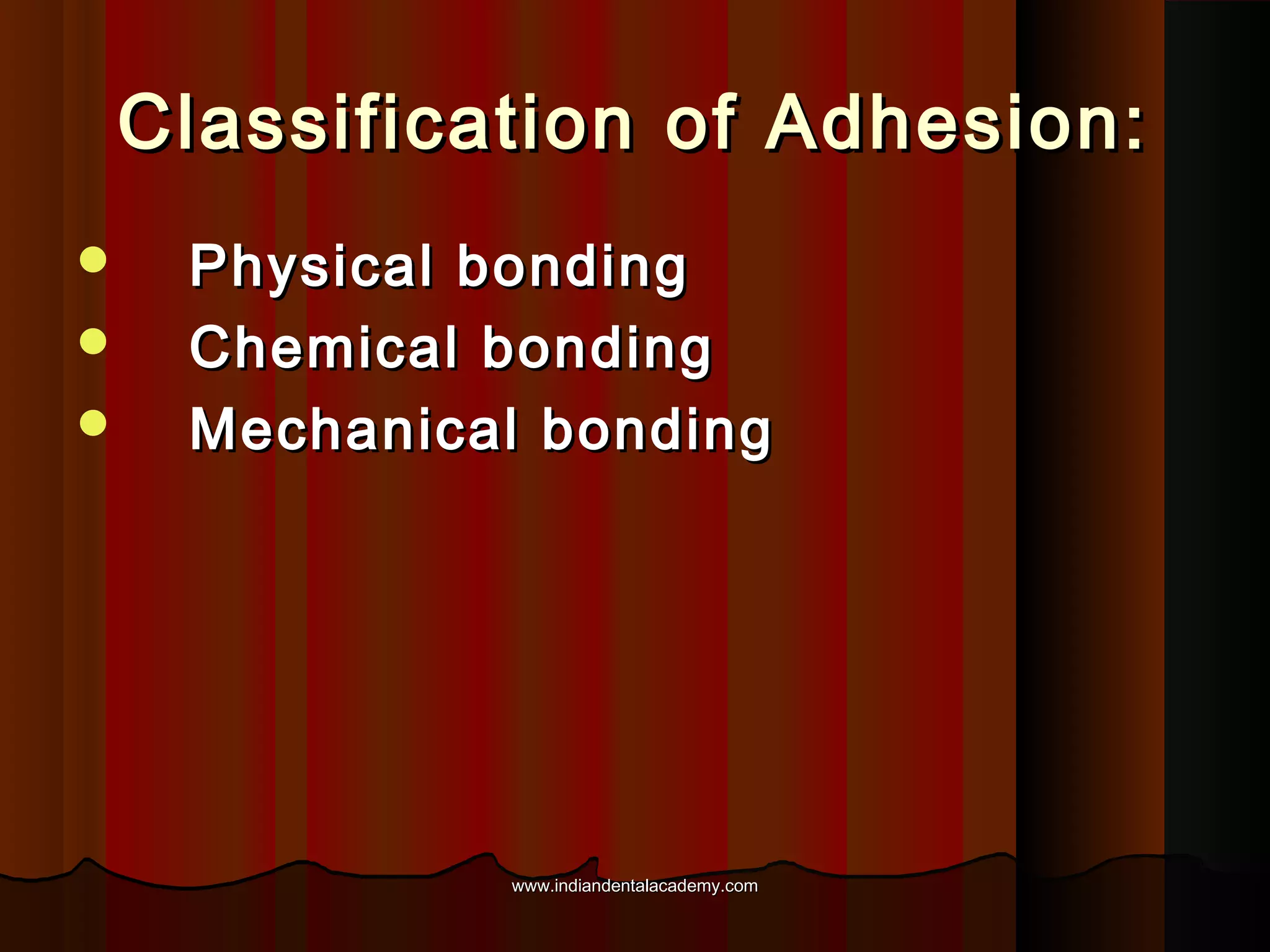
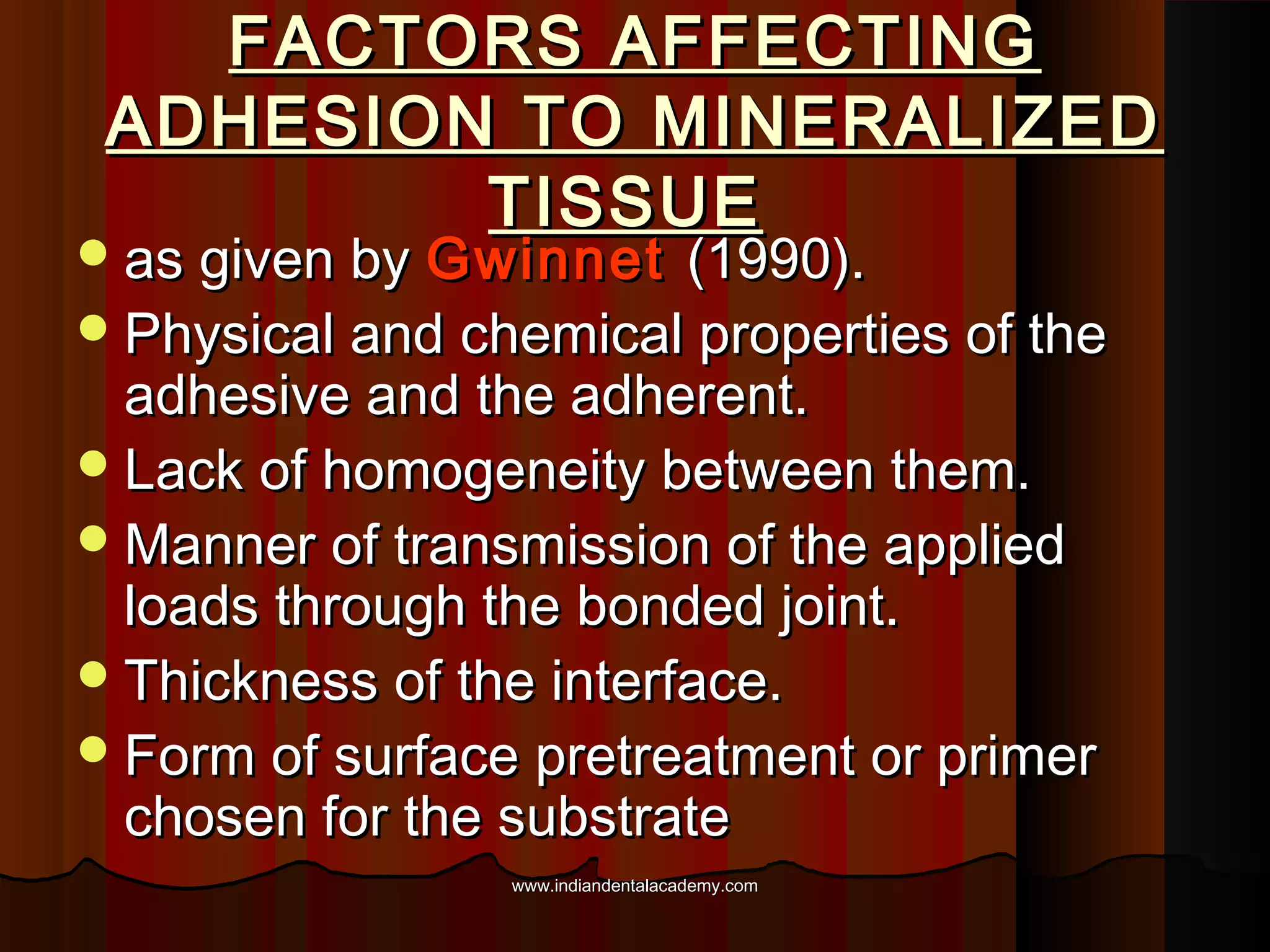
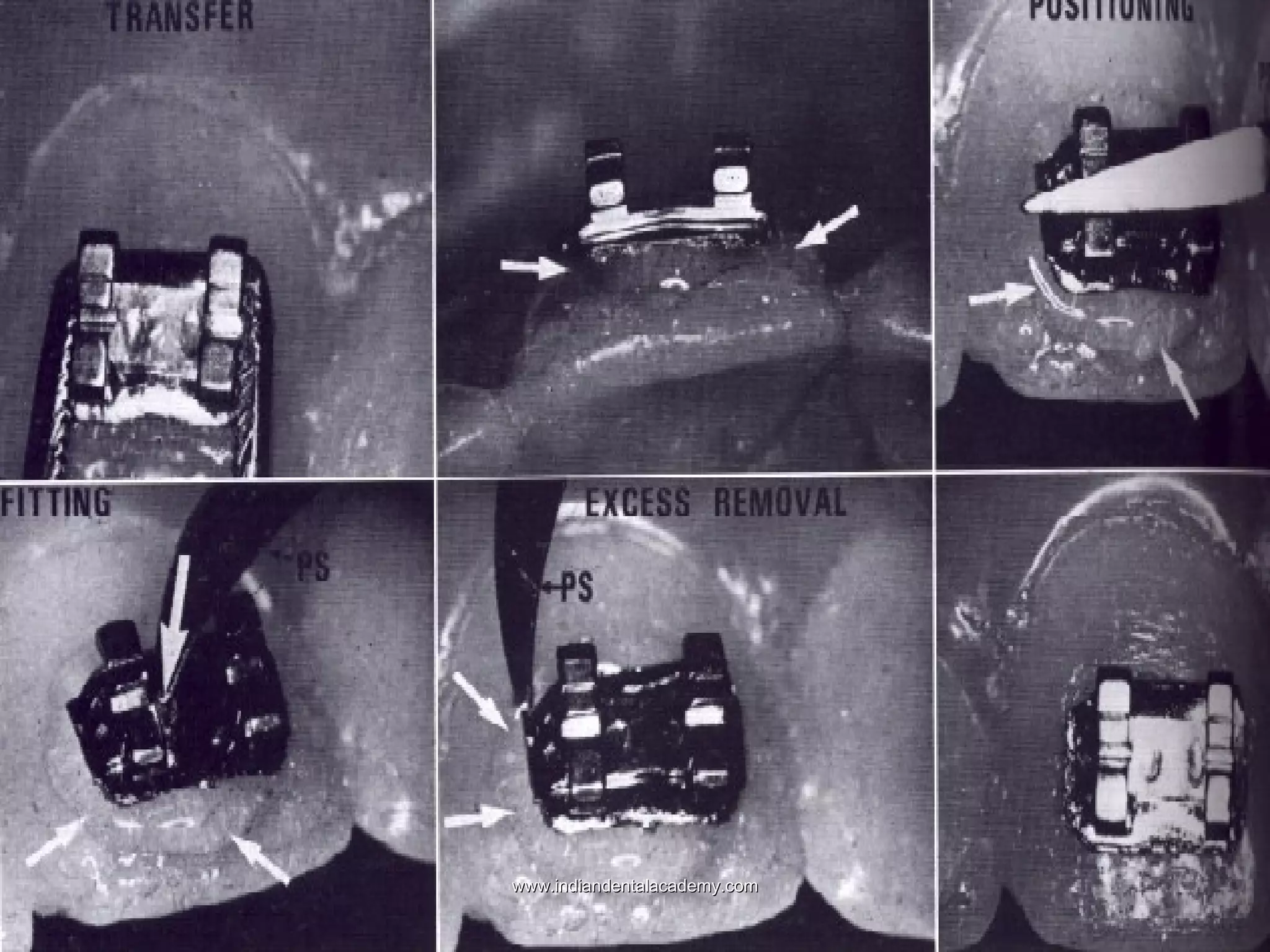
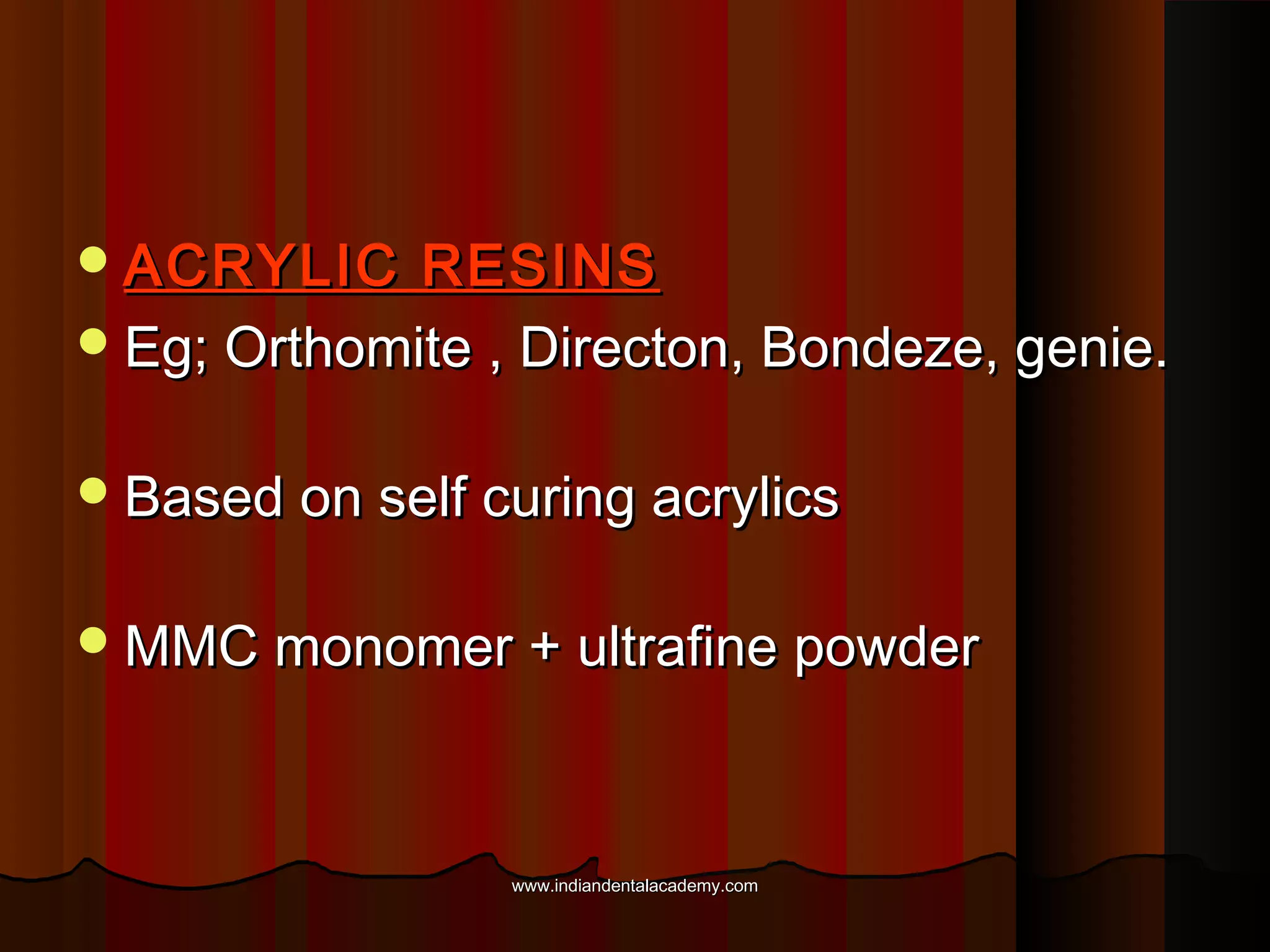
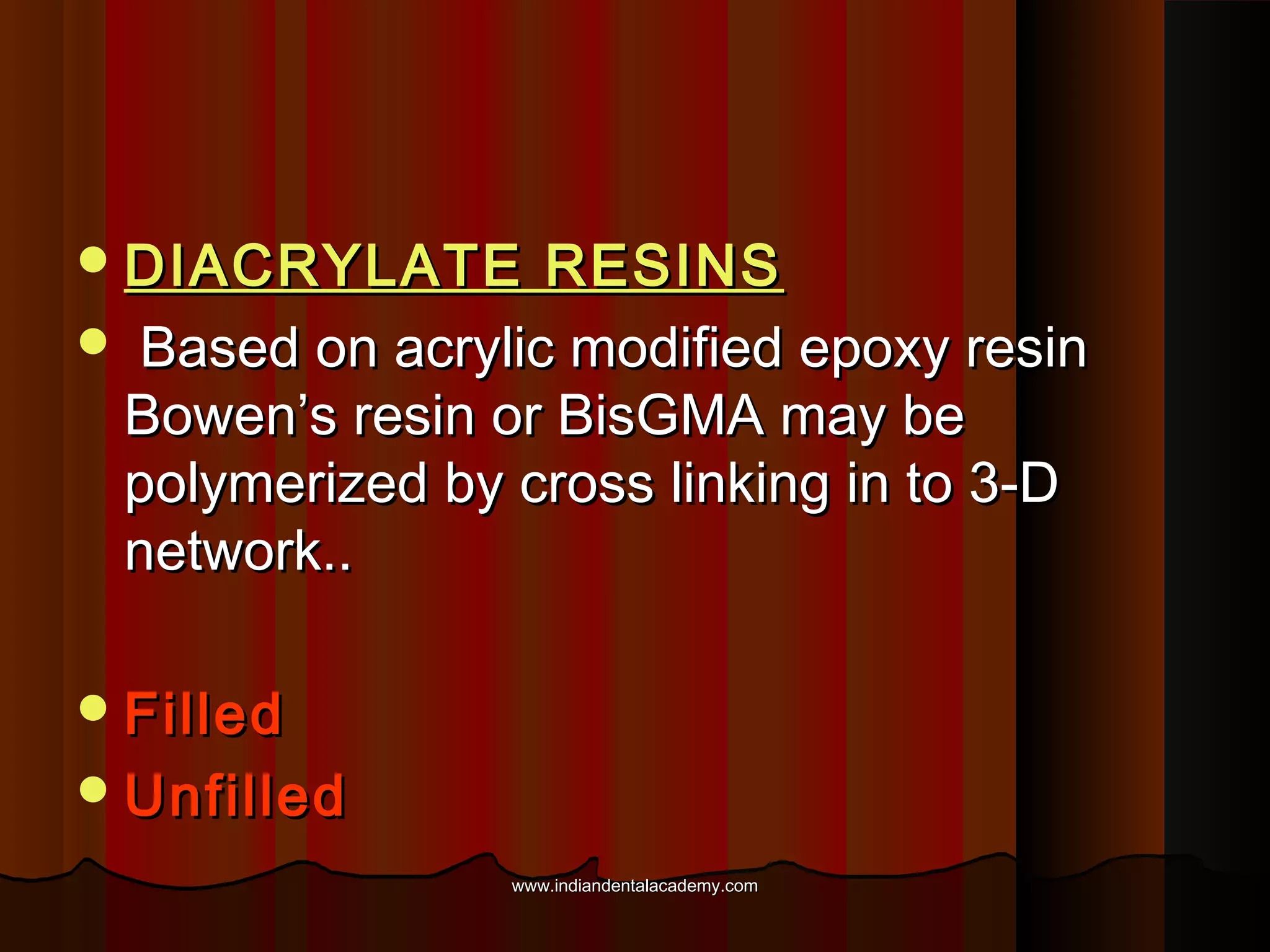
The document discusses the evolution of adhesive bonding techniques in dentistry, particularly emphasizing the acid-etch bonding method pioneered by Michael Buonocore in 1955. It details various materials used for direct bonding, including composites and glass ionomers, as well as the chemistry behind synthetic resins and their polymerization processes. Key terminologies related to dental adhesion, bonding agents, and the nature of enamel are also defined to provide a comprehensive understanding of the topic.