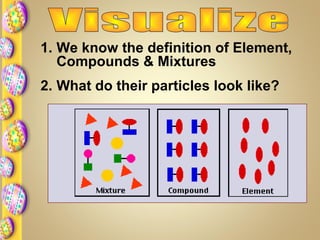
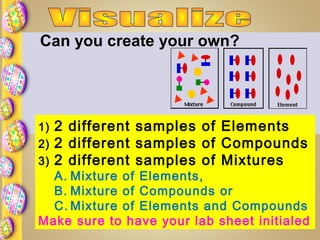
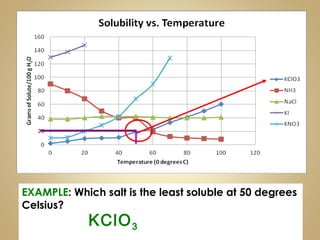
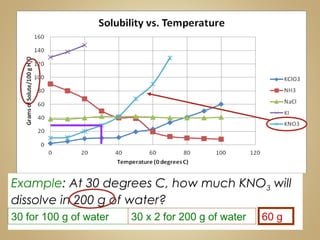
This document contains instructions for a science lesson on elements, compounds, and mixtures. Students are asked to:
1) Complete notes and an initialed lab worksheet on the differences between elements, compounds, and mixtures.
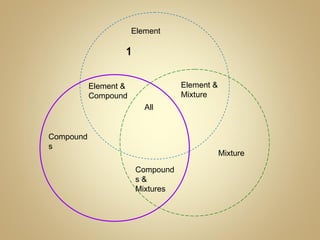
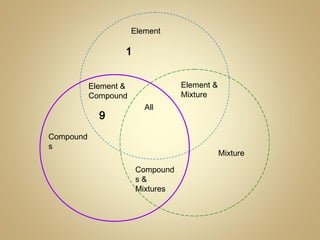
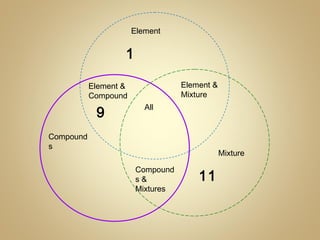
2) Finish a Venn diagram comparing elements, compounds, and mixtures.
3) Turn in the completed notes and lab worksheet the next day along with STAR cards for a quiz on Friday.