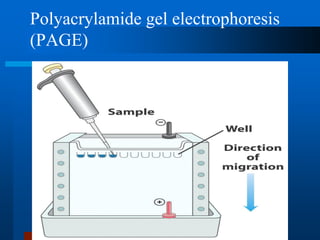
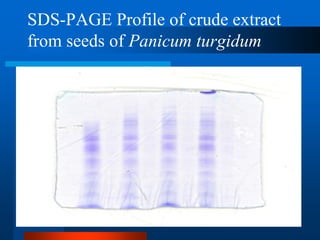
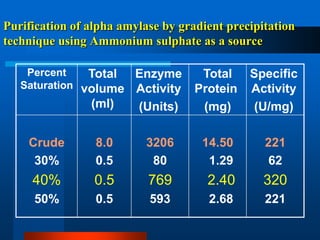
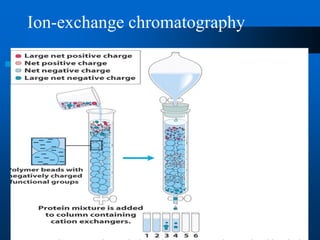
This document provides information on electrophoresis techniques. It discusses how electrophoresis separates charged molecules like proteins and nucleic acids using an electric current. The key techniques covered are:
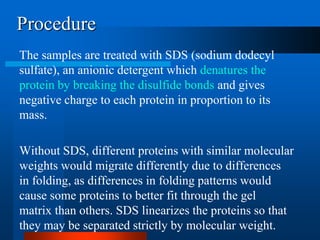
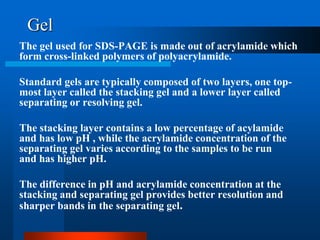
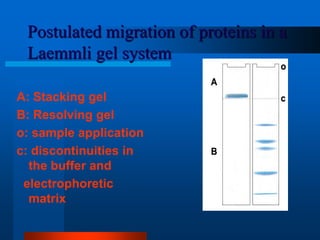
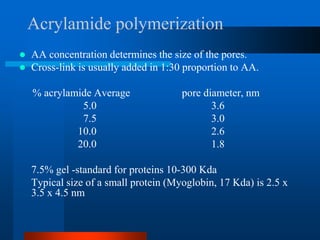
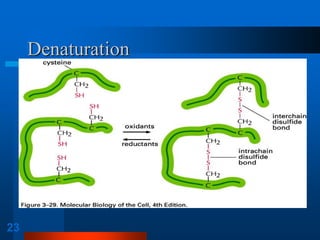
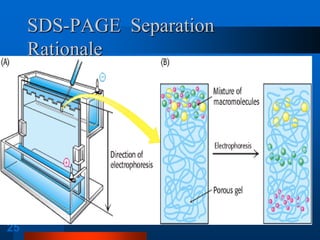
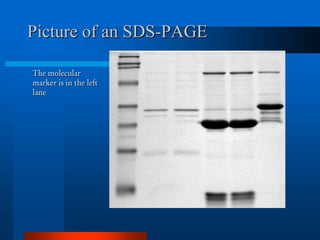
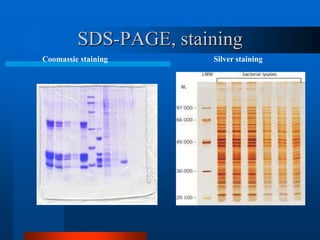
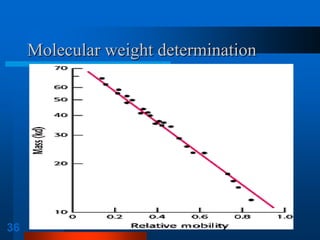
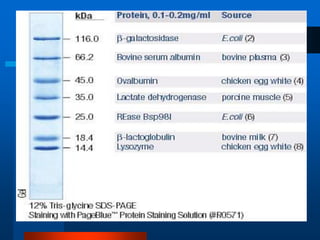
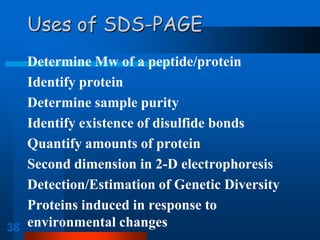
1. SDS-PAGE, which uses sodium dodecyl sulfate to denature proteins and give them a uniform negative charge for separation by size in a polyacrylamide gel.
2. Native PAGE, which separates intact proteins by their charge-to-size ratio.
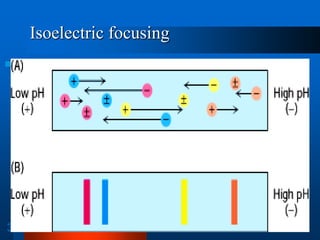
3. Isoelectric focusing, which separates proteins based on their isoelectric point in a pH gradient gel.
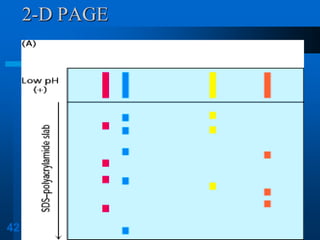
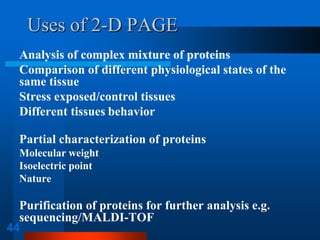
It also discusses two-dimensional electrophoresis, which combines isoelectric focusing and SDS-PAGE to better resolve complex protein mixtures. The document