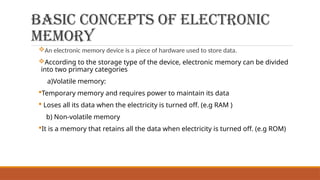
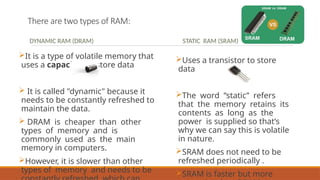
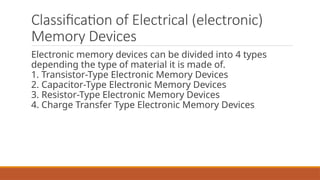
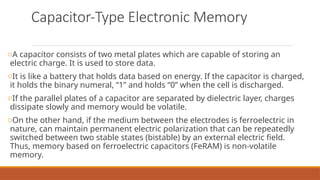
This document discusses the fundamental concepts of electronic memory systems, categorizing them into volatile and non-volatile memory types, including RAM, ROM, and various secondary storage options. It also covers classifications of electronic memory devices based on materials and functionalities, such as transistor, capacitor, resistor, and charge transfer types, as well as organic and hybrid memory devices. Additionally, the document explains the application of photoactive and electroactive materials in optoelectronic devices and the nature and applications of liquid crystals.























![Organic materials for Optoelectronic devices [Light absorbing materials –
Polythiophenes -P3HT]
Polythiophenes are environmentally and thermally stable material.
Properties:
Semiconducting polymer with high stability and exhibits conductivity due to holes therefore
considered as p-type semiconductor.
Great capability as light-absorbing materials.
P3HT has a crystalline structure and good charge-transport(conductivity) properties
required for Optoelectronics.
Fundamental bandgap of P3HT is 490nm visible region, corresponding to π →π*
transition, giving electron-hole pair.
Used in photovoltaic devices, lithium battery,fabrication of memory devices.](https://image.slidesharecdn.com/module2-1-240922043421-d9f08aaa/85/ElectronicsbasicandgoodhitMODULE2-1-pptx-28-320.jpg)











