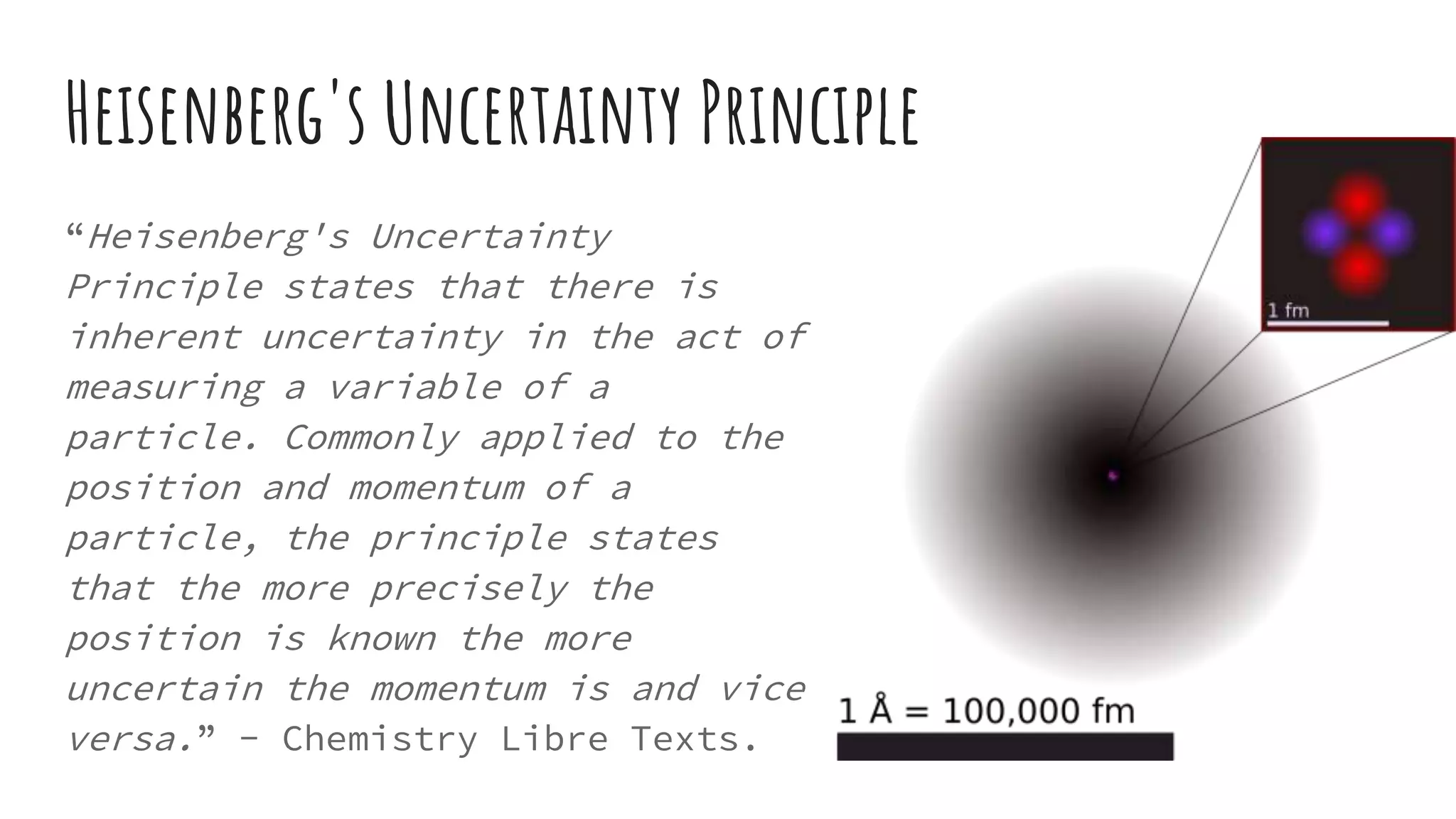
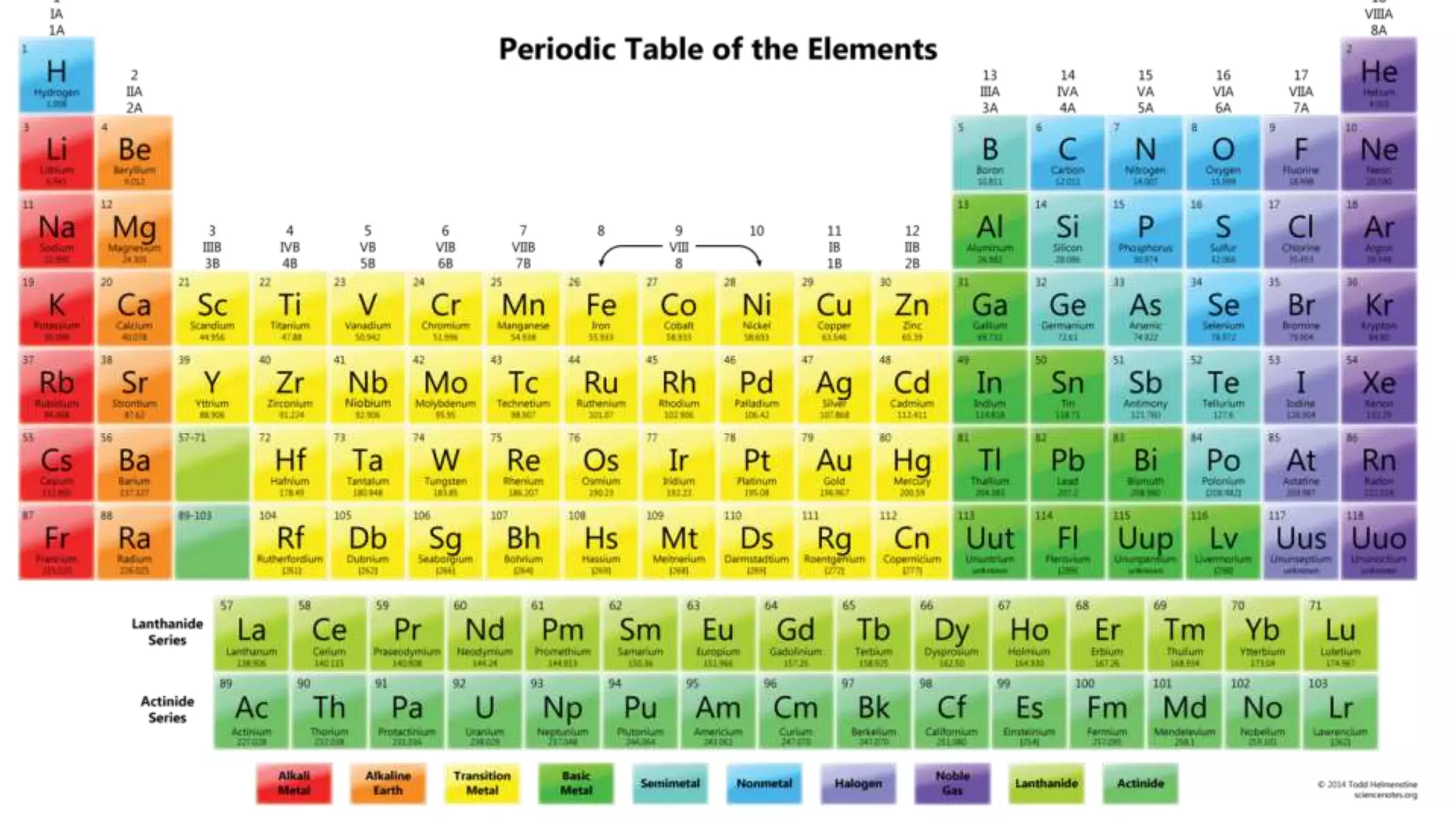
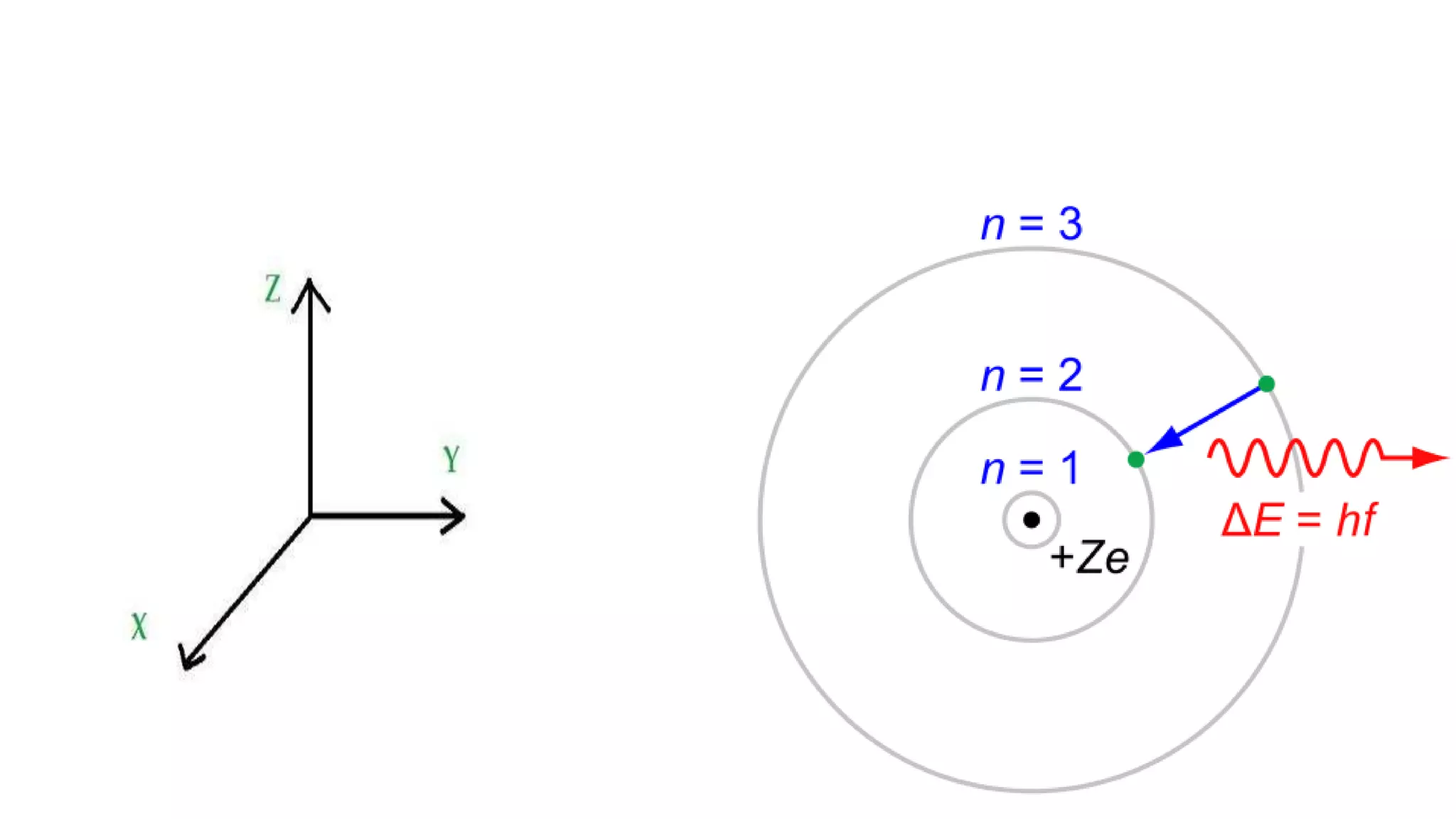
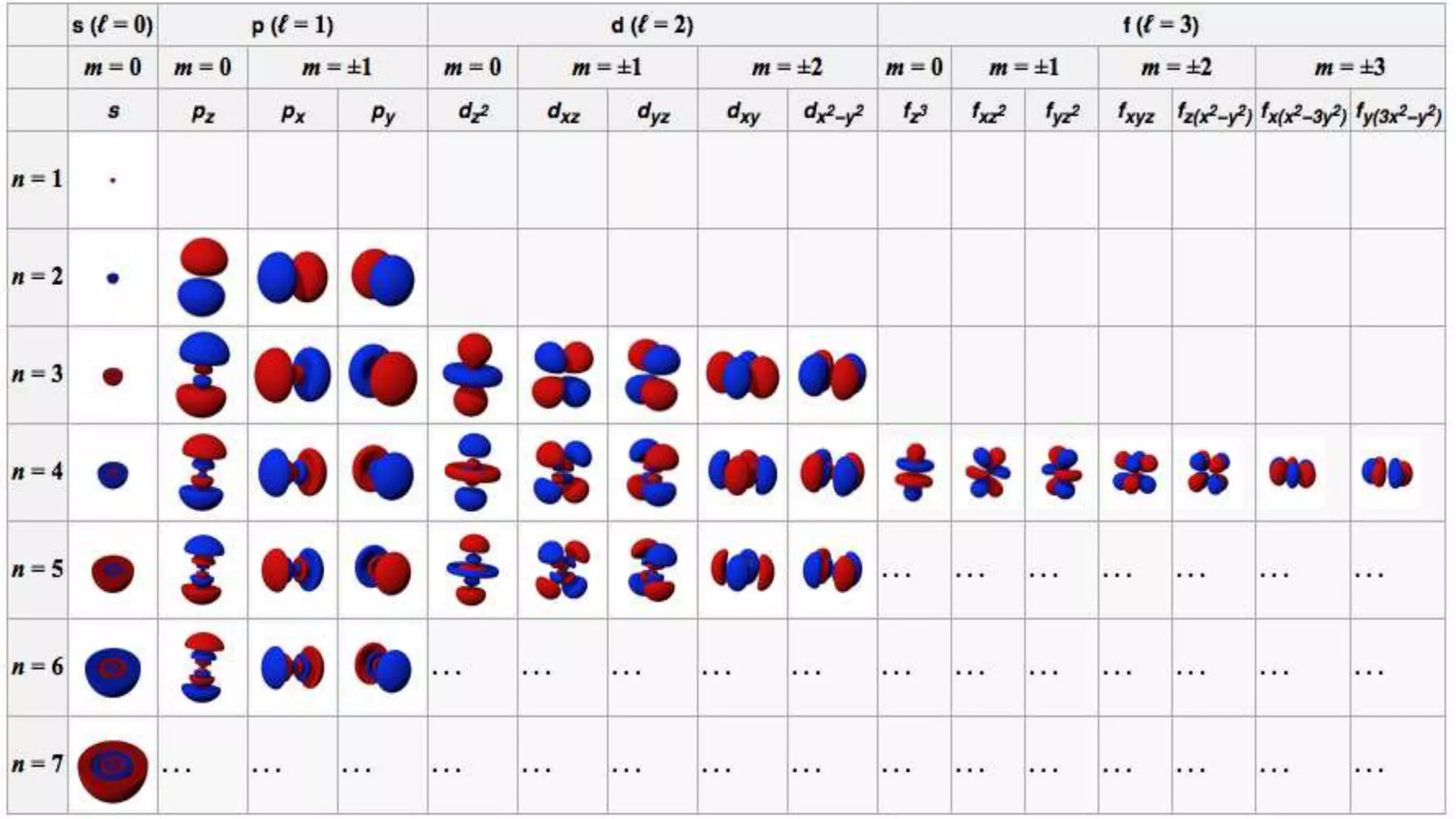
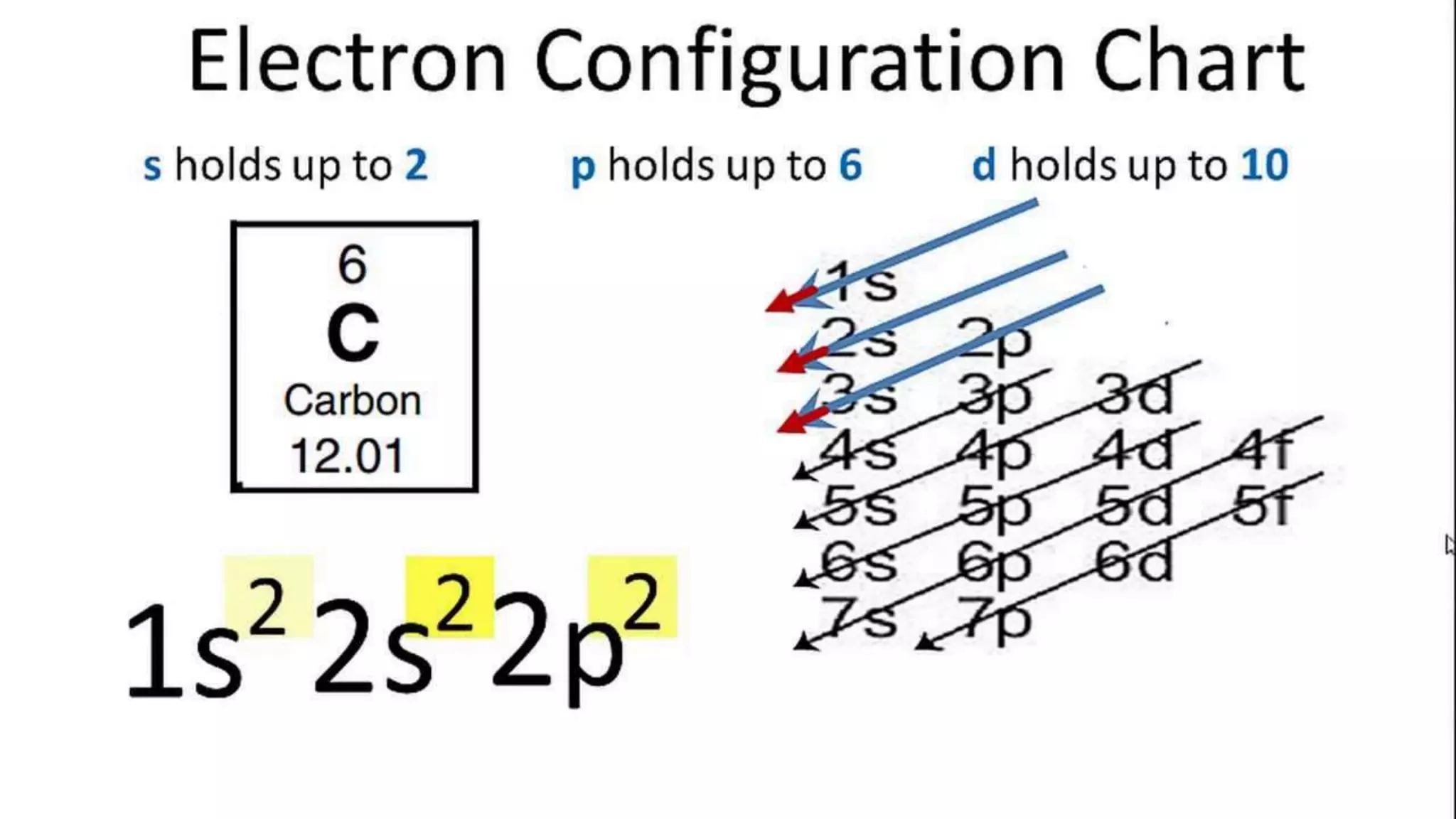
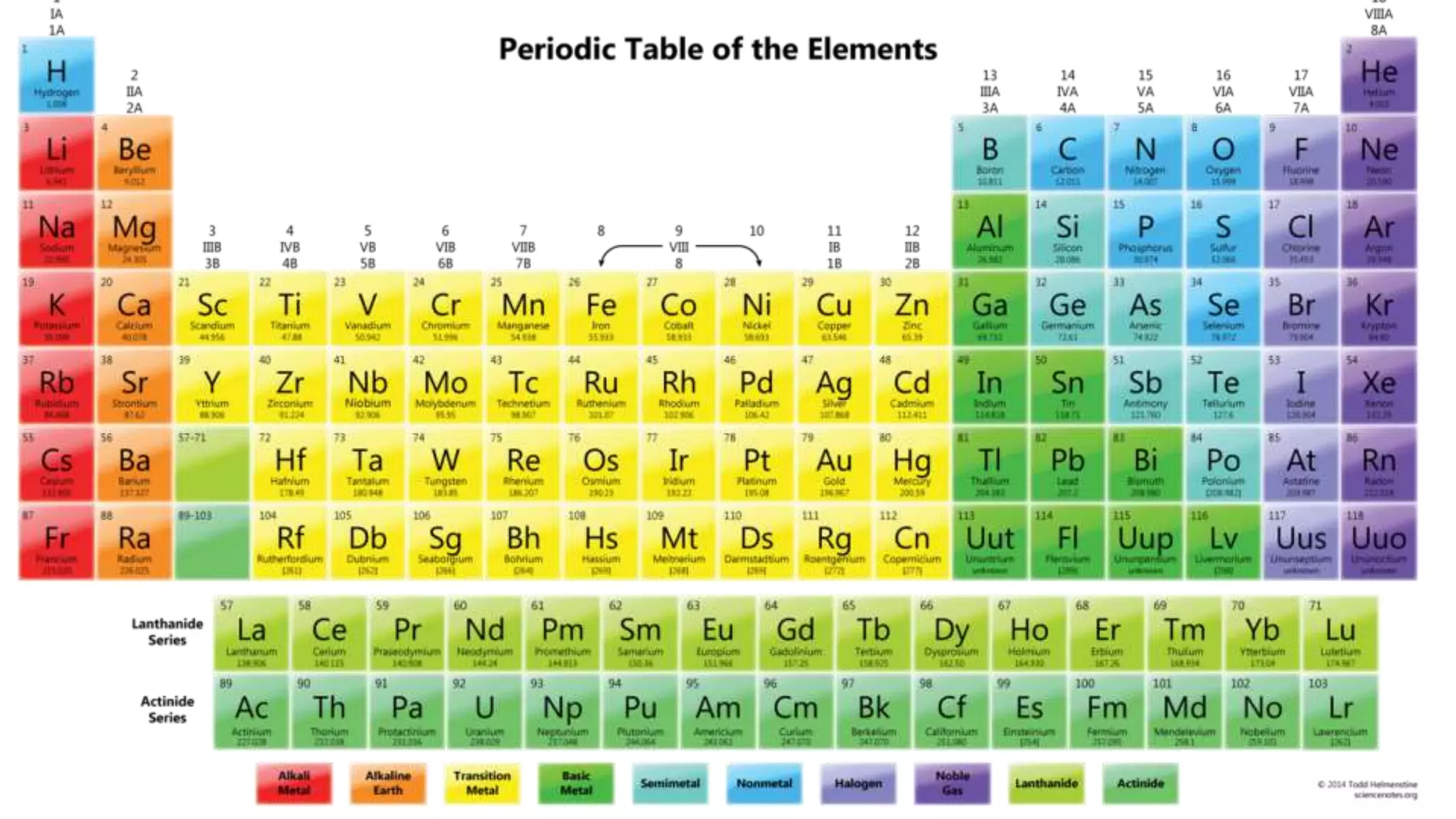
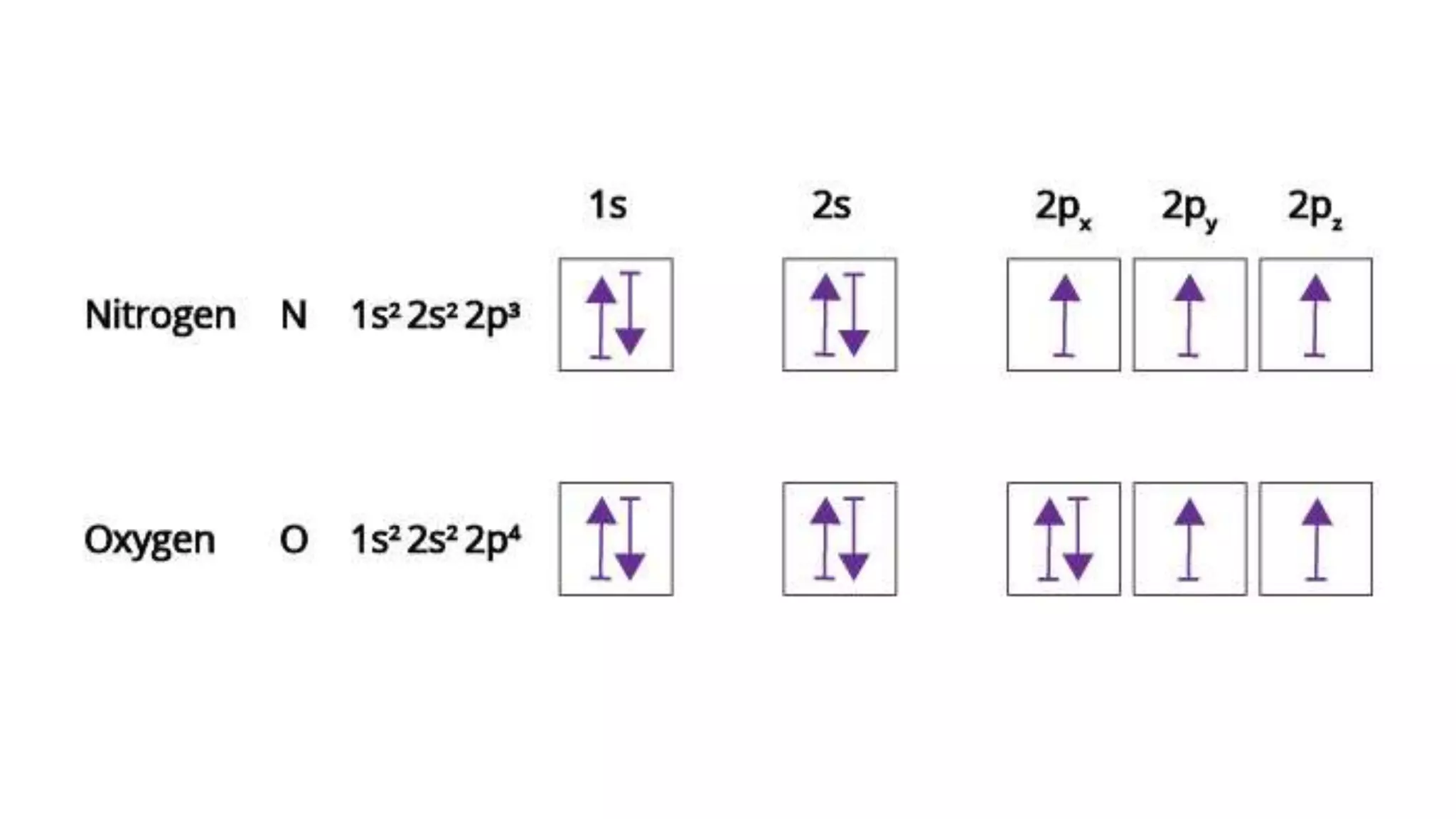
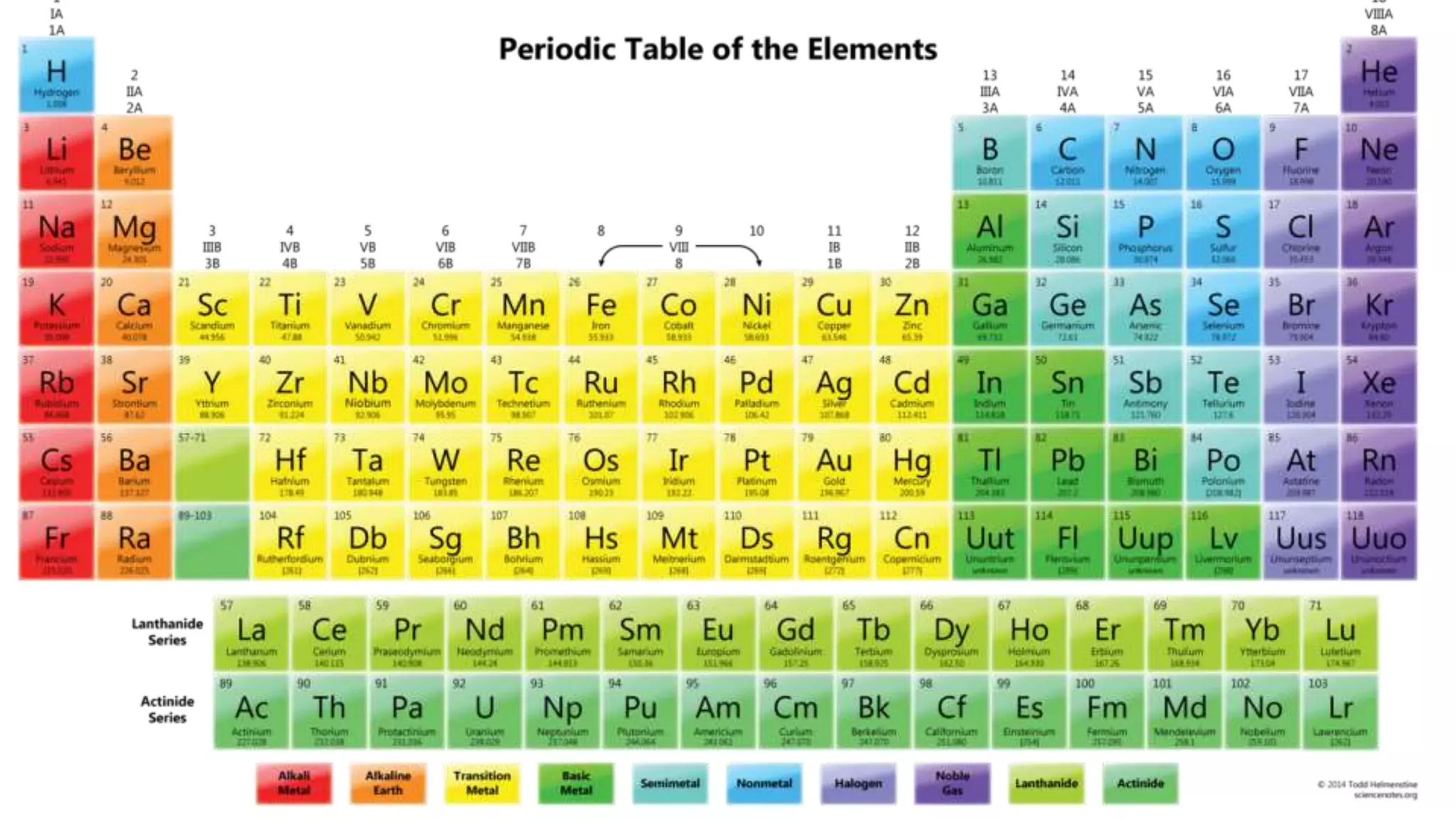
This document discusses electron probability and orbitals, the periodic table, and Heisenberg's Uncertainty Principle. Heisenberg's Uncertainty Principle states that there is inherent uncertainty in measuring a particle's variable, such that measuring its position precisely makes its momentum less certain, and vice versa, according to a quote from Chemistry Libre Texts.