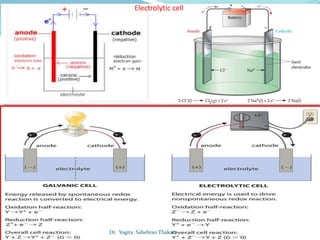
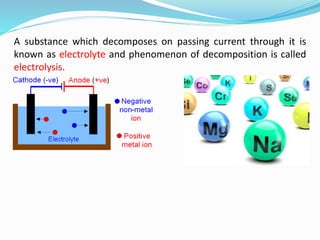
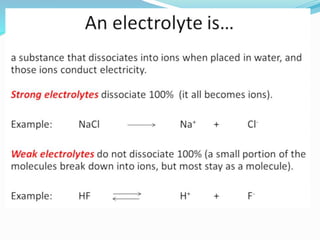
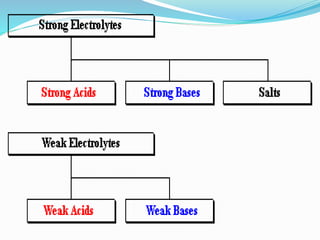
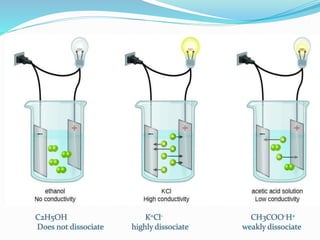
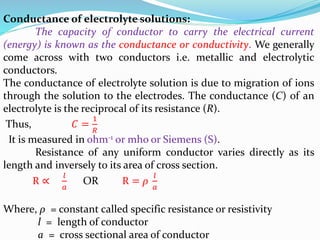
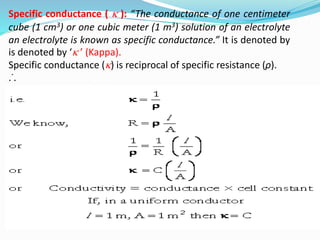
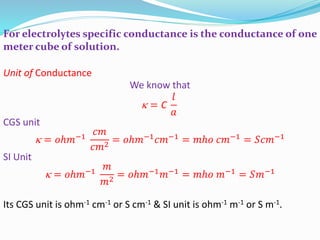
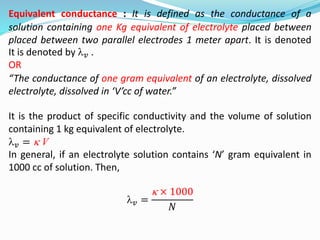
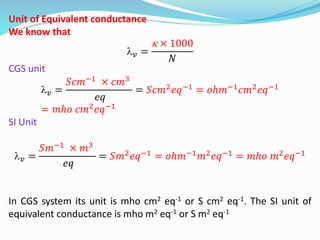
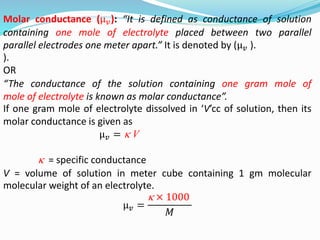
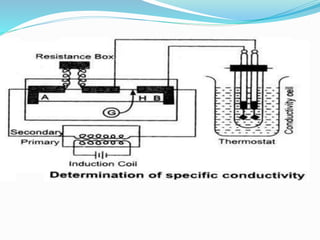
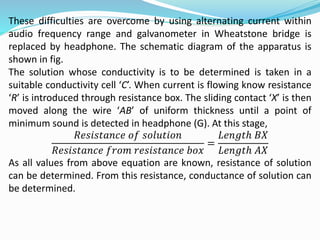
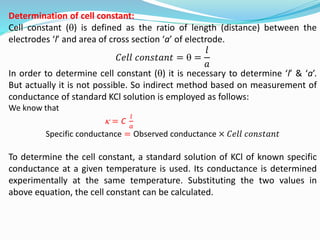
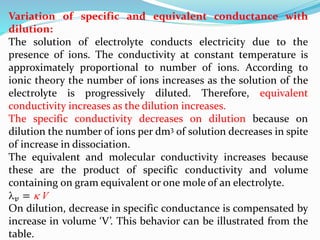
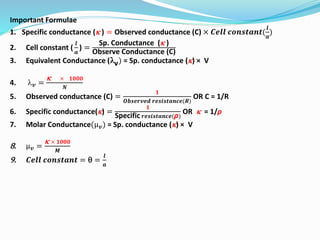
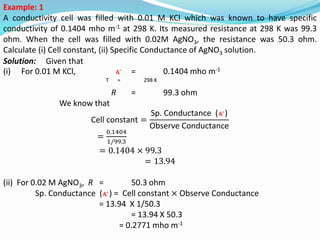
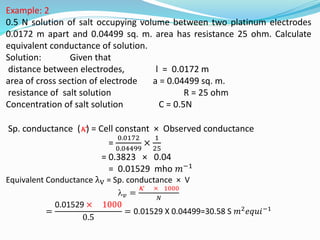
The document discusses electrochemistry, focusing on concepts such as conductance, specific conductance, equivalent conductance, and molar conductance, along with their definitions and formulas. It explains how to determine conductance using the Wheatstone bridge method and details the behavior of conductance with dilution. Various examples are provided to illustrate calculations related to specific and equivalent conductance in electrolytic solutions.