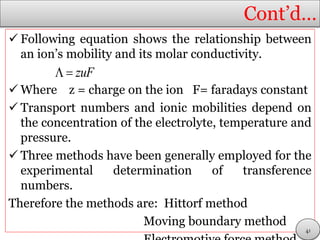
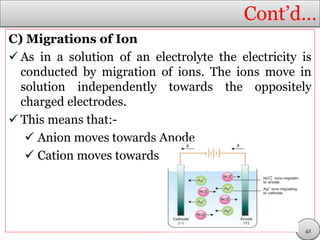
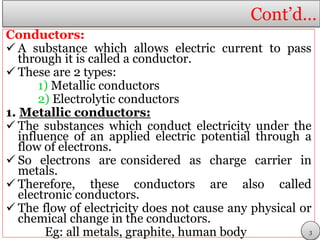
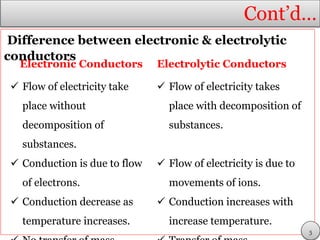
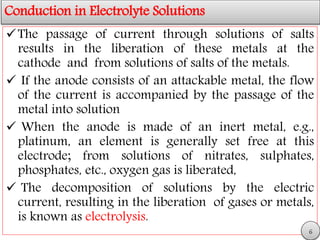
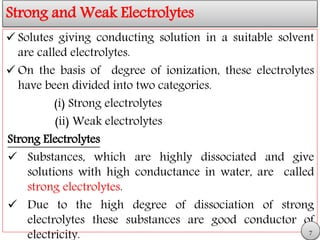
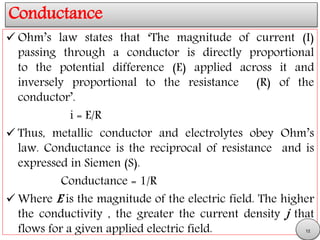
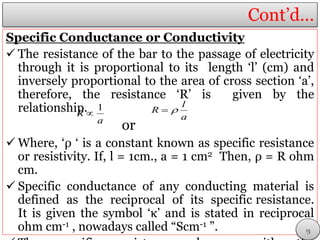
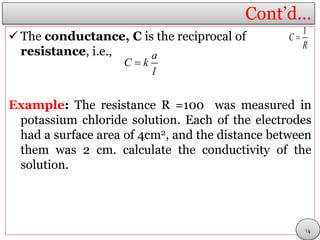
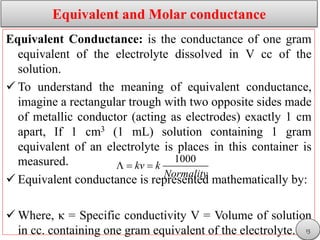
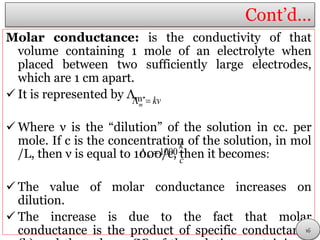
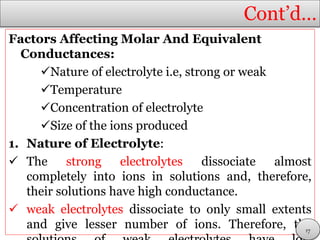
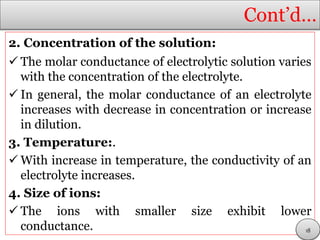
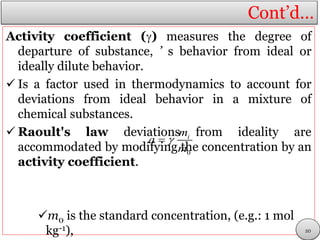
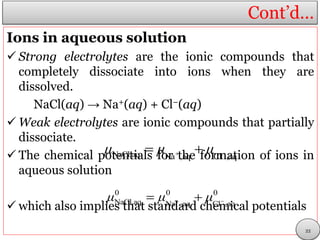
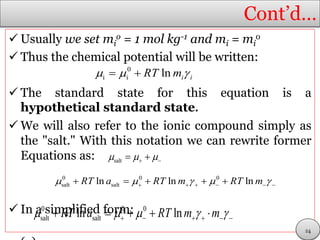
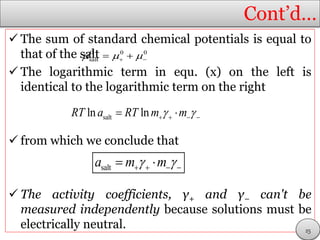
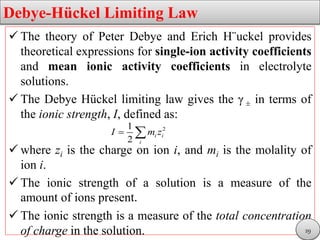
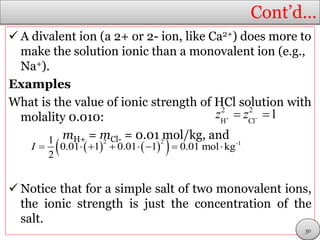
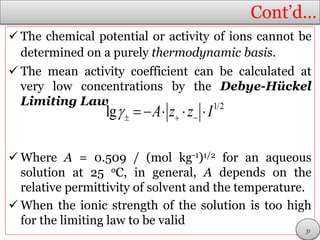
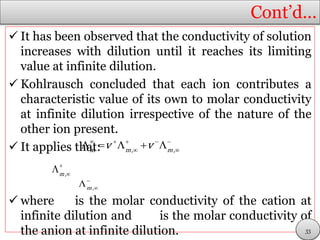
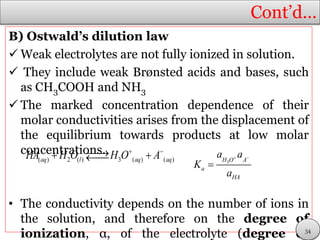
This document discusses electrolytic solutions and electrochemistry. It begins by defining electrochemistry as the study of chemical reactions involving electron transfer between an electrode and electrolyte. It then discusses different types of solutions, distinguishing between electrolytic and non-electrolytic solutions. Electrolytic solutions contain ions and are electrically conductive. The document also discusses the differences between electronic and electrolytic conductors, and how conductivity is affected by various factors like temperature, concentration, and ion size. It introduces concepts like equivalent conductance, molar conductance, activity, and activity coefficients. In summary, the document provides an overview of key concepts relating to electrolytic solutions and electrochemistry.








![Cont’d…
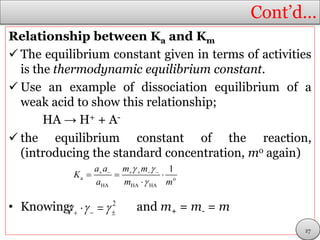
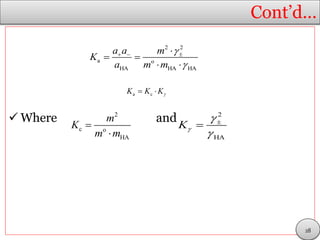
It is defined so that, for the acid HA at a molar
concentration c, at equilibrium.
If we ignore activity coefficients, the acidity
constant, Ka, is approximately
(x)
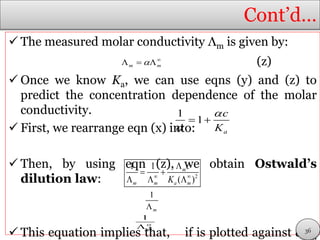
From which it follows that:
(y)
The acid is fully deprotonated at infinite dilution,
and its molar conductivity is then
3[ ]H O c
[ ]A c
[ ] (1 )HA c
2
( )
1
a
c
K
1
2
4
{(1 ) 1}
2
a
a
K c
c K
m
35](https://image.slidesharecdn.com/chapter1ppt-200407062349/85/Chapter-1-ppt-36-320.jpg)