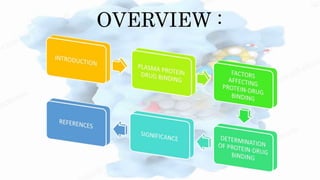
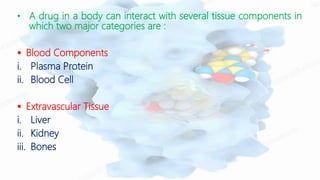
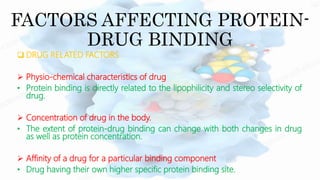
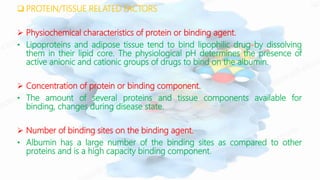
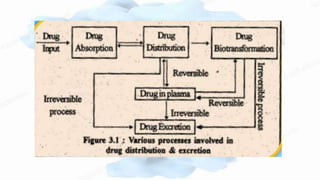
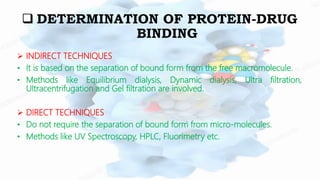
The document discusses drug-protein interactions, highlighting their significance in determining drug activity, distribution, and toxicity in the body. It describes various types of protein-drug binding, primarily focusing on plasma proteins like albumin, α-1 acid glycoprotein, lipoproteins, and globulins. Additionally, it covers factors affecting these interactions and techniques for determining protein-drug binding, emphasizing their clinical relevance in absorption, elimination, and therapeutic targeting.