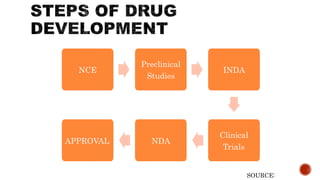
The document outlines the stages of drug development, including preclinical studies, clinical trials, and the regulatory approval process. It emphasizes the importance of clinical data management in generating reliable data, as well as the role of the regulatory affairs department in obtaining and maintaining product approvals. Upon FDA approval, a drug enters post-marketing surveillance, requiring periodic safety updates from the manufacturer.
![DRUG
DEVELOPMENT
BY _
VIKAS
KHEDKAR
[ R.C.PATEL INSTITUTE OF
PHARMACY, SHIRPUR ]](https://image.slidesharecdn.com/drugdevelopment-230123174616-3c86a6e9/85/DRUG-DEVELOPMENT-pptx-1-320.jpg)







![MAIN WORK OF
RA
I] TO PREPARE CTD/ ECTD.
Itincludes,,,
Module1tomodule5.
Most important module is module 3
It is nothing but the CMC/ ICH.M4
Module 3 composed of drug substance and drug product formulation.
To Prepare Complete dossier.
what is dossier?
It involves ------
👉 3.2.S & 3.2.P
SOURCE:](https://image.slidesharecdn.com/drugdevelopment-230123174616-3c86a6e9/85/DRUG-DEVELOPMENT-pptx-10-320.jpg)



