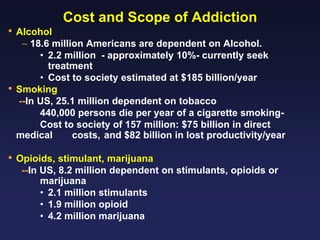
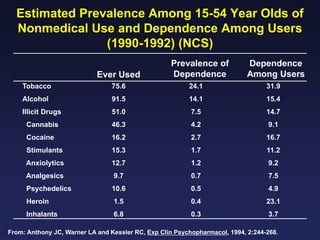
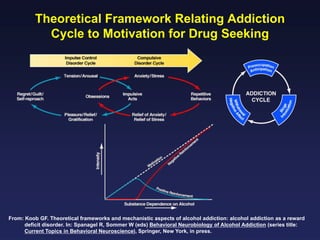
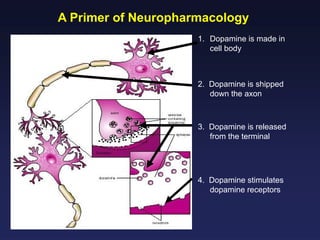
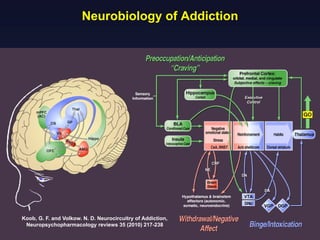
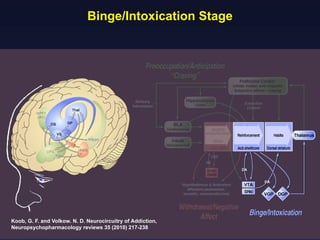
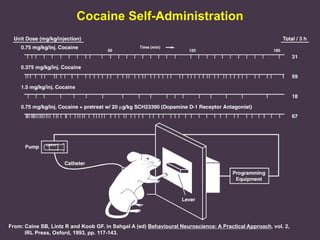
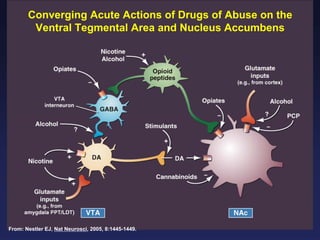
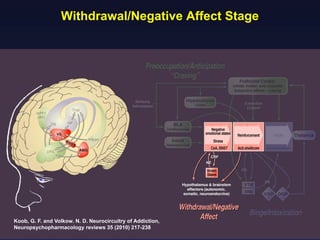
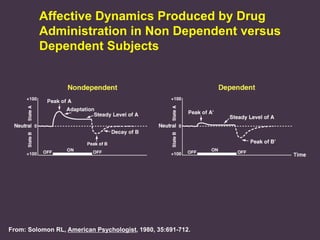
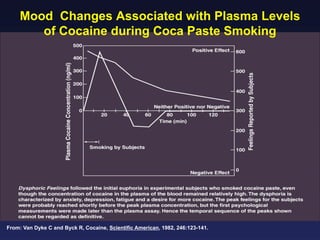
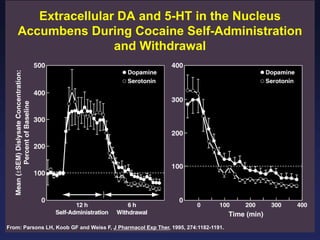
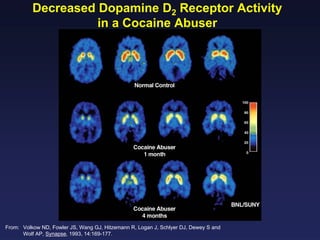
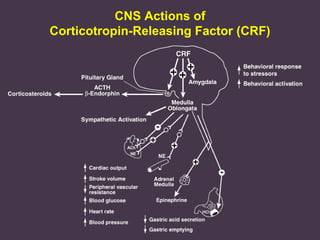
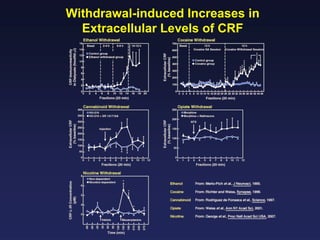
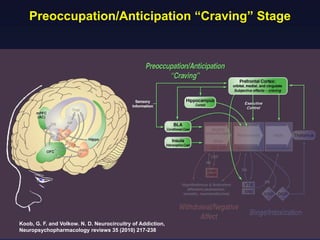
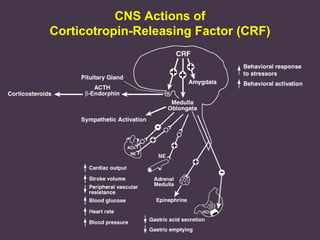
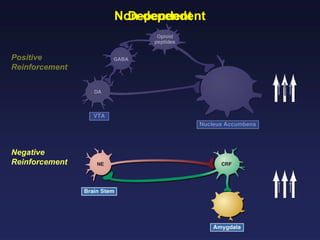
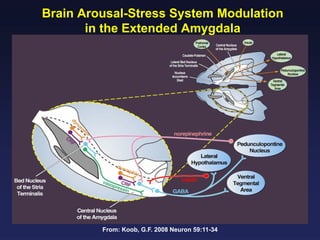
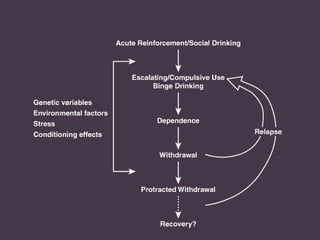
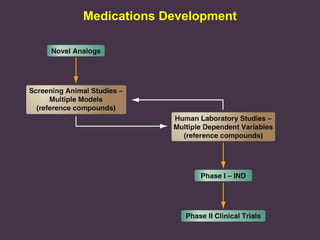
This document provides an overview of addiction and the neuroscience of addiction. It begins by defining addiction as a chronically relapsing disorder characterized by compulsion to seek a stimulus, loss of control, and negative emotional state during withdrawal. It discusses compulsive drug use and the neurobiology of the binge/intoxication, withdrawal/negative affect, and preoccupation/craving stages of addiction. It provides data on the prevalence and societal costs of various addictions. It also summarizes current and potential medications to treat the positive and negative reinforcing aspects of addiction.