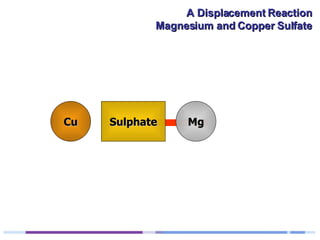
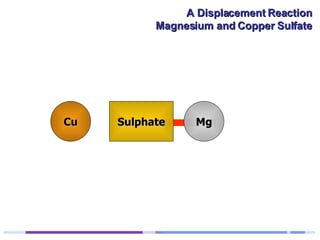
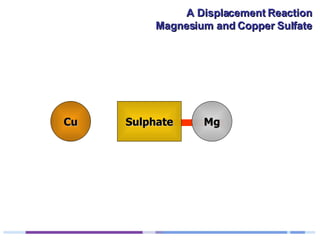
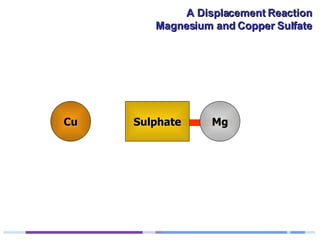
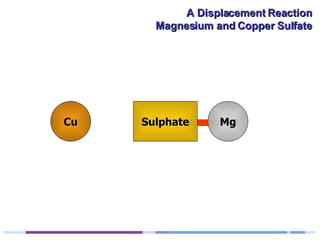
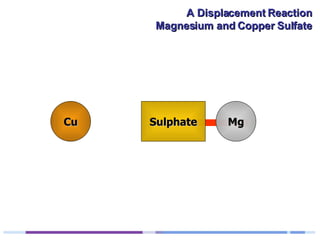
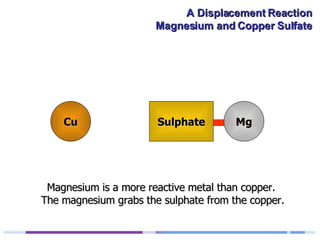
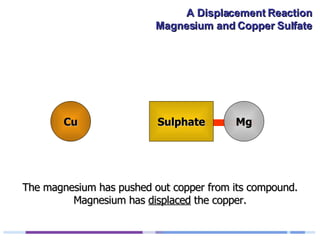
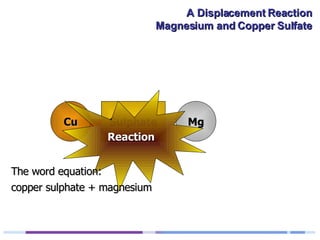
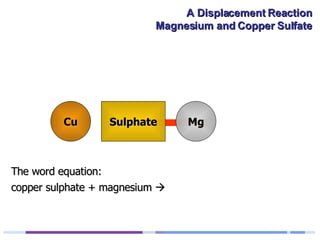
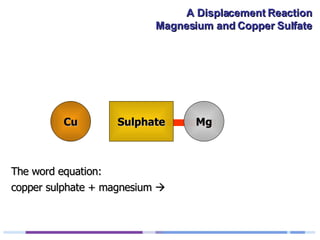
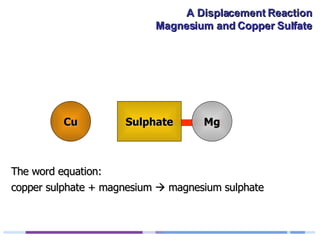
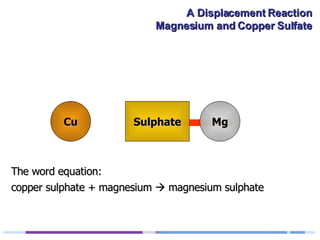
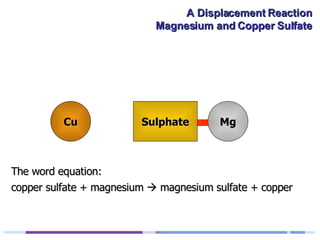
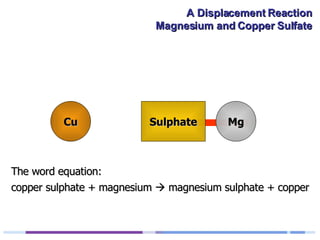
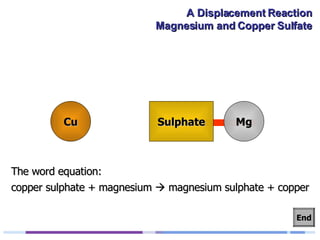
A solution of copper sulphate was placed on a spotting tile and magnesium powder was added. Over ten minutes, the solution fizzed and turned colorless as a brown solid formed at the bottom, indicating a replacement reaction between the magnesium and copper sulphate that produced magnesium sulphate and copper. The word equation summarizing the reaction is: copper sulphate + magnesium → magnesium sulphate + copper.