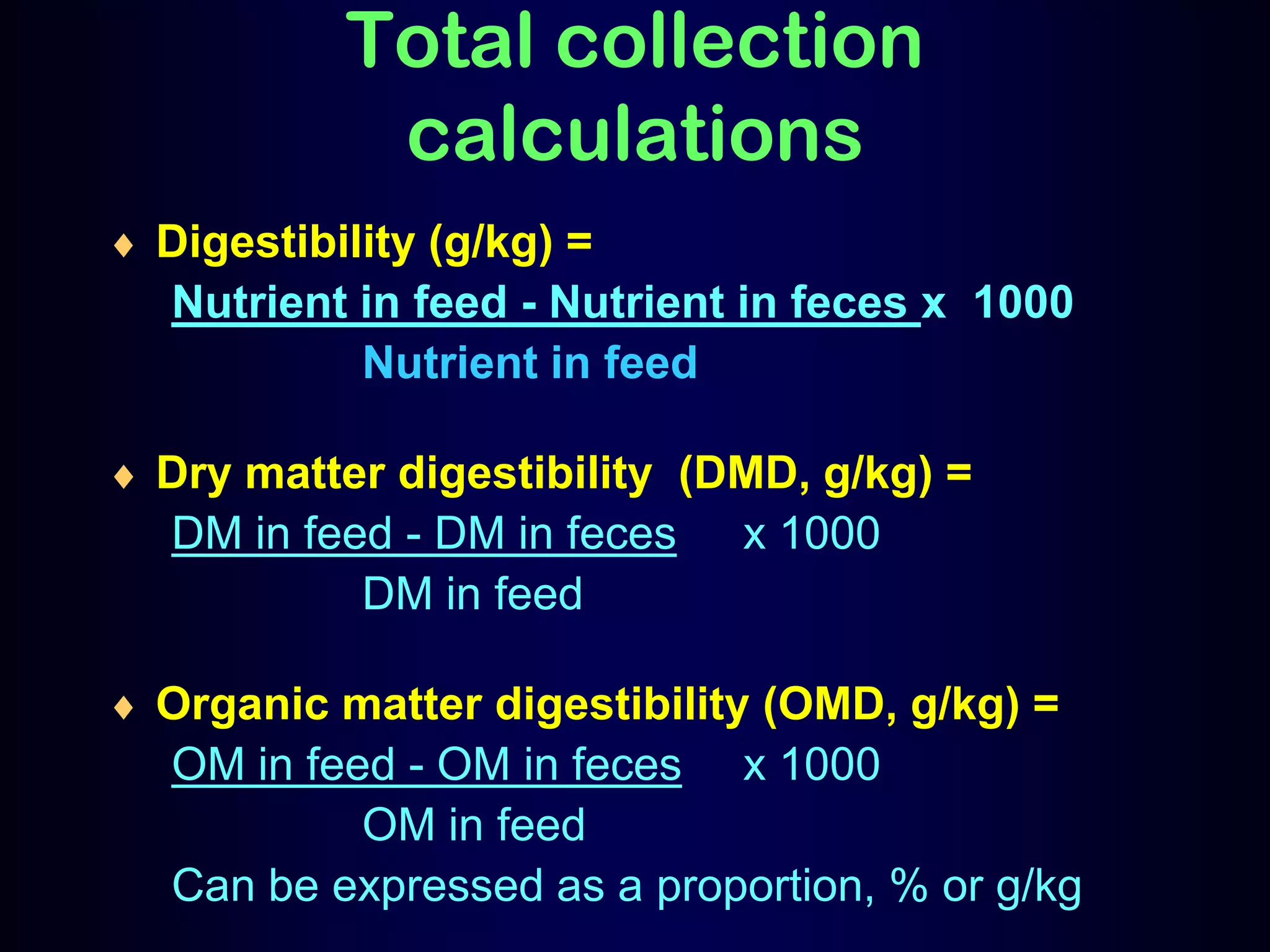
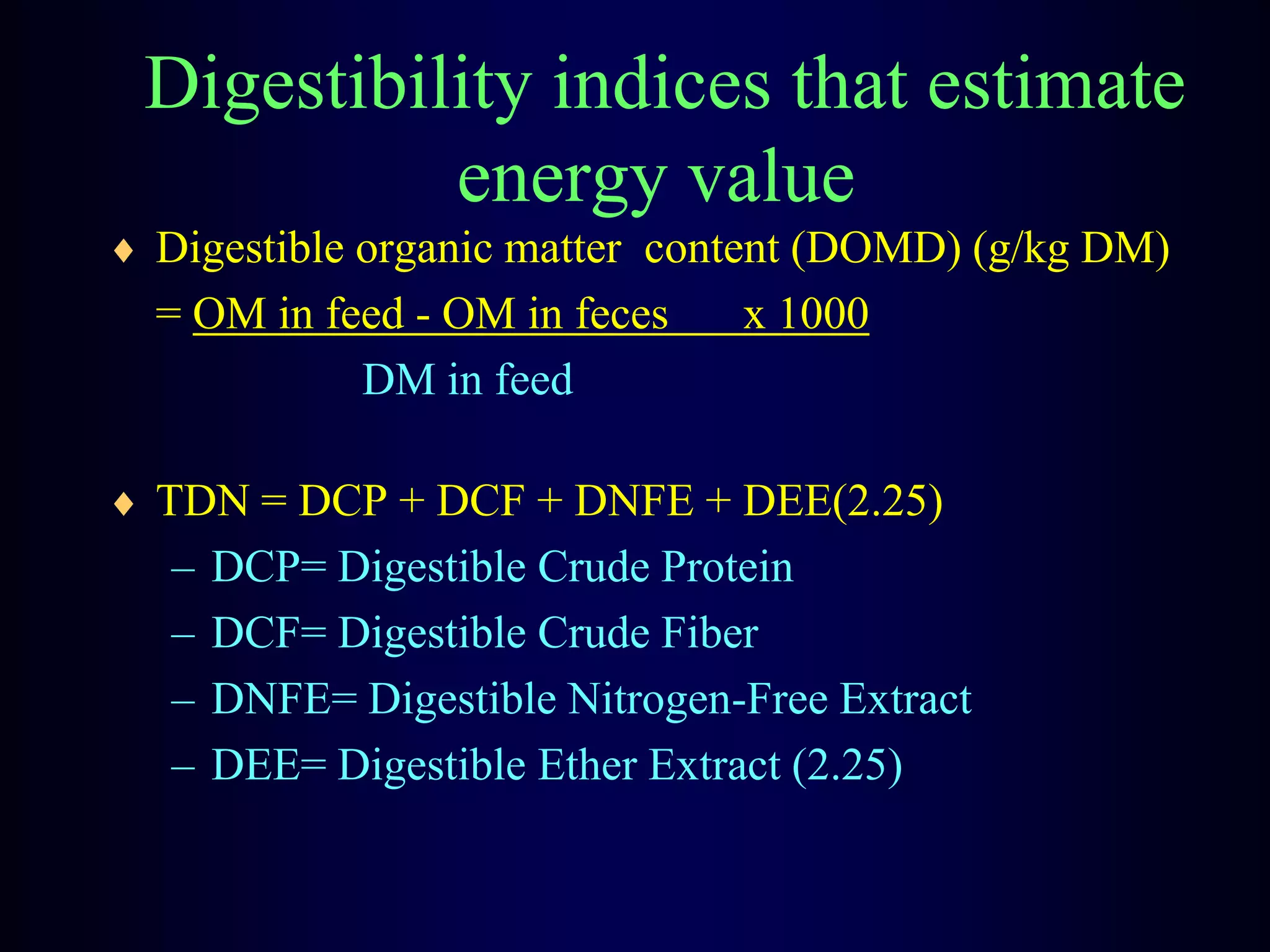
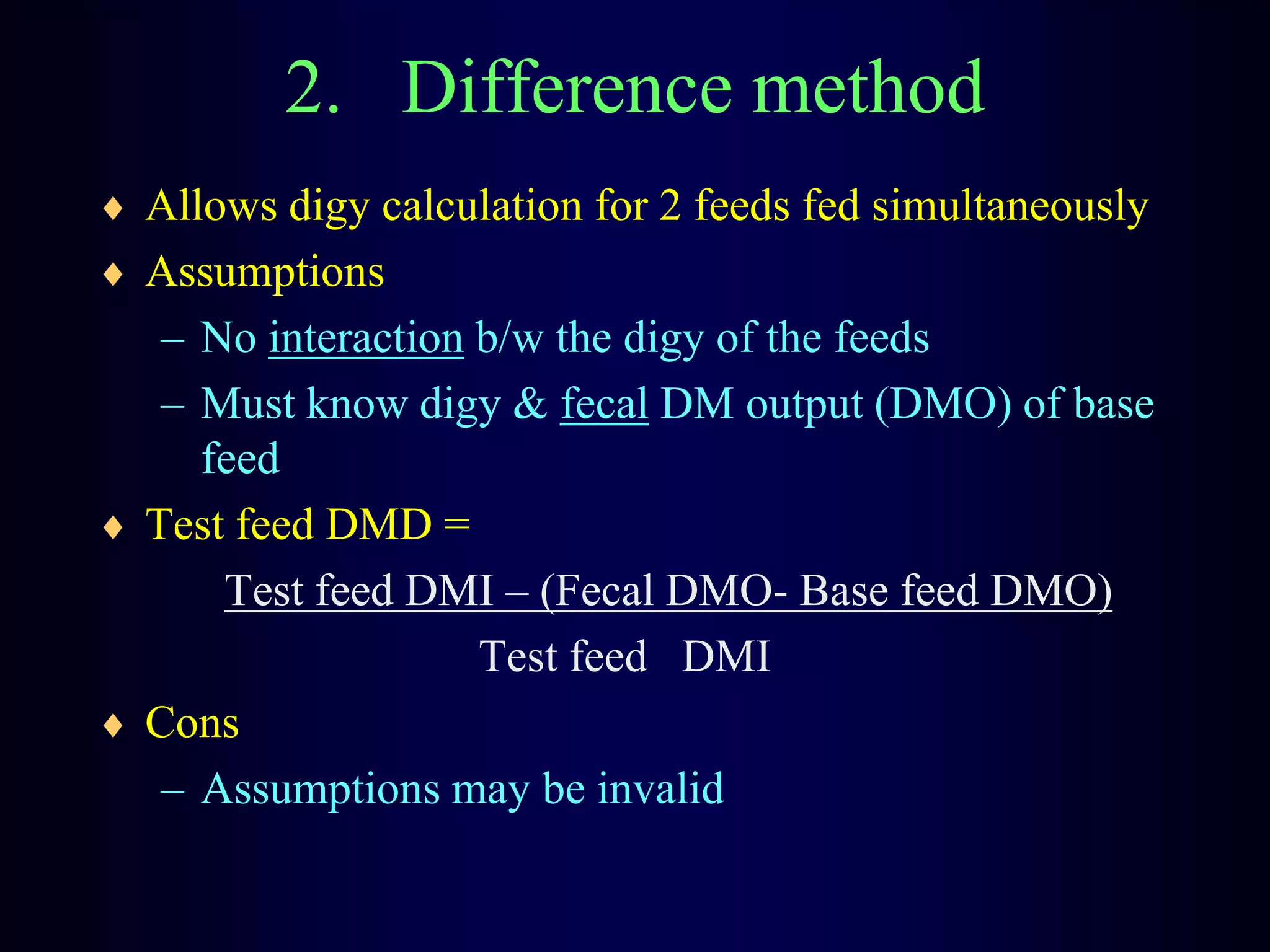
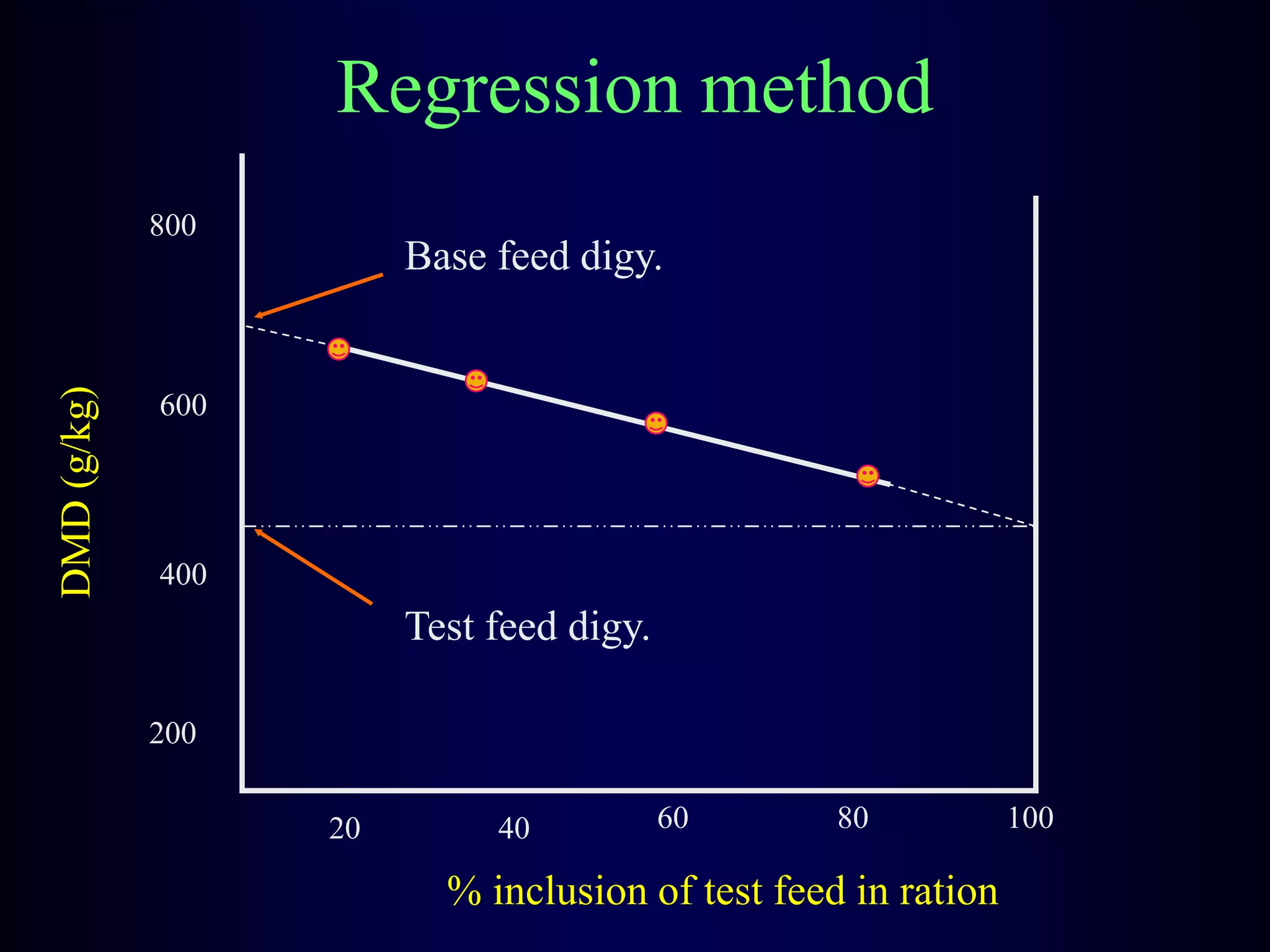
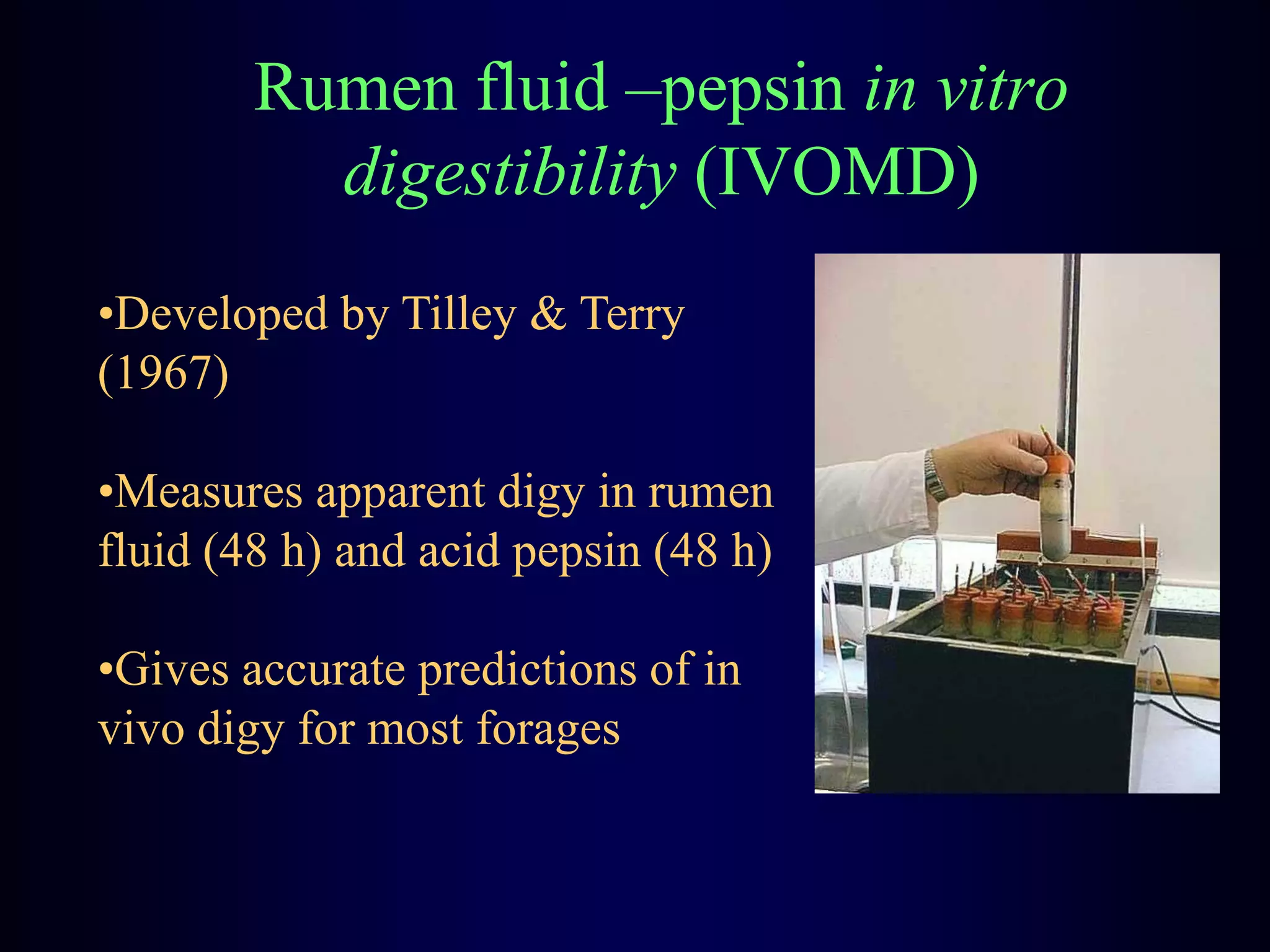
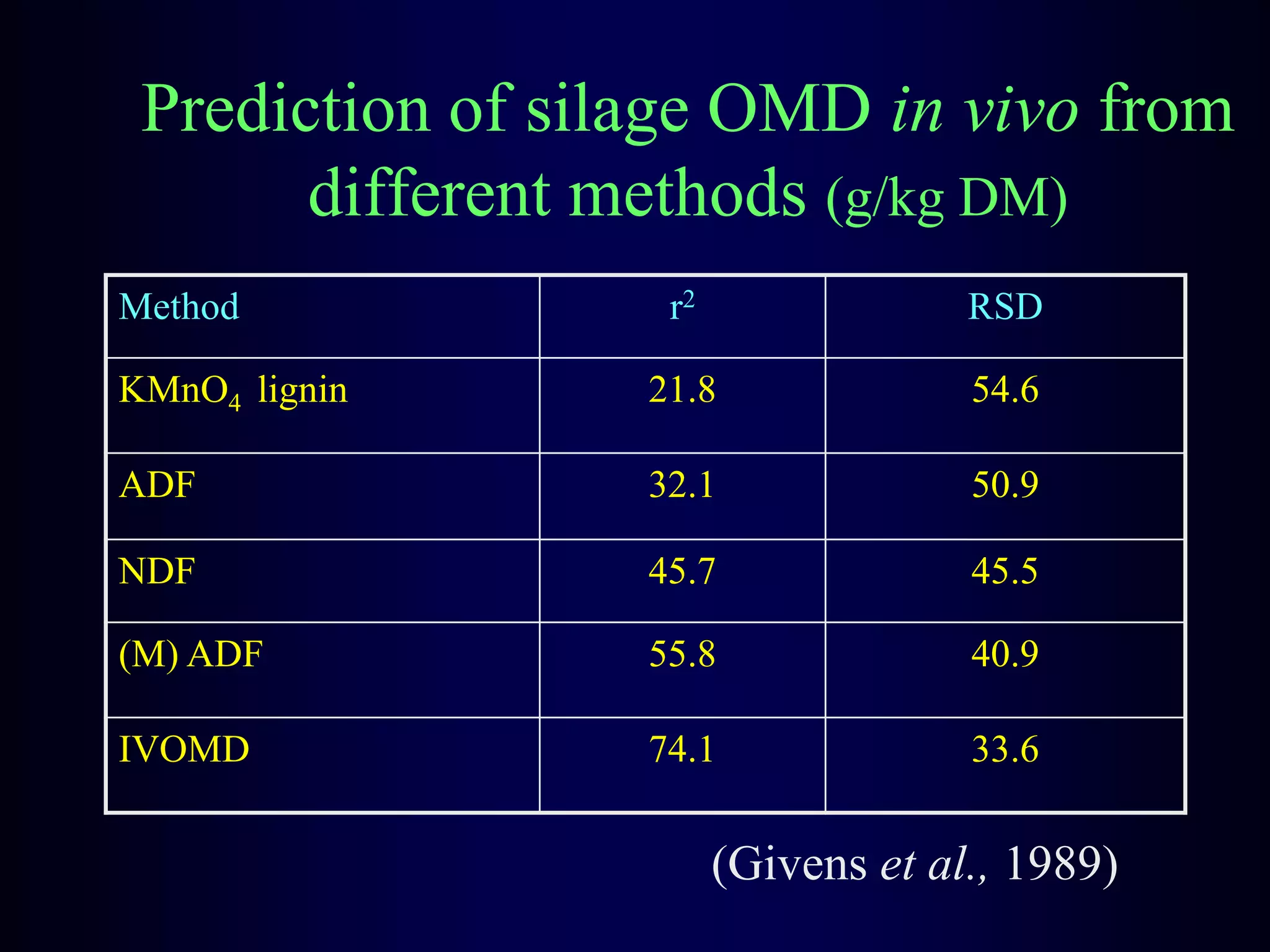
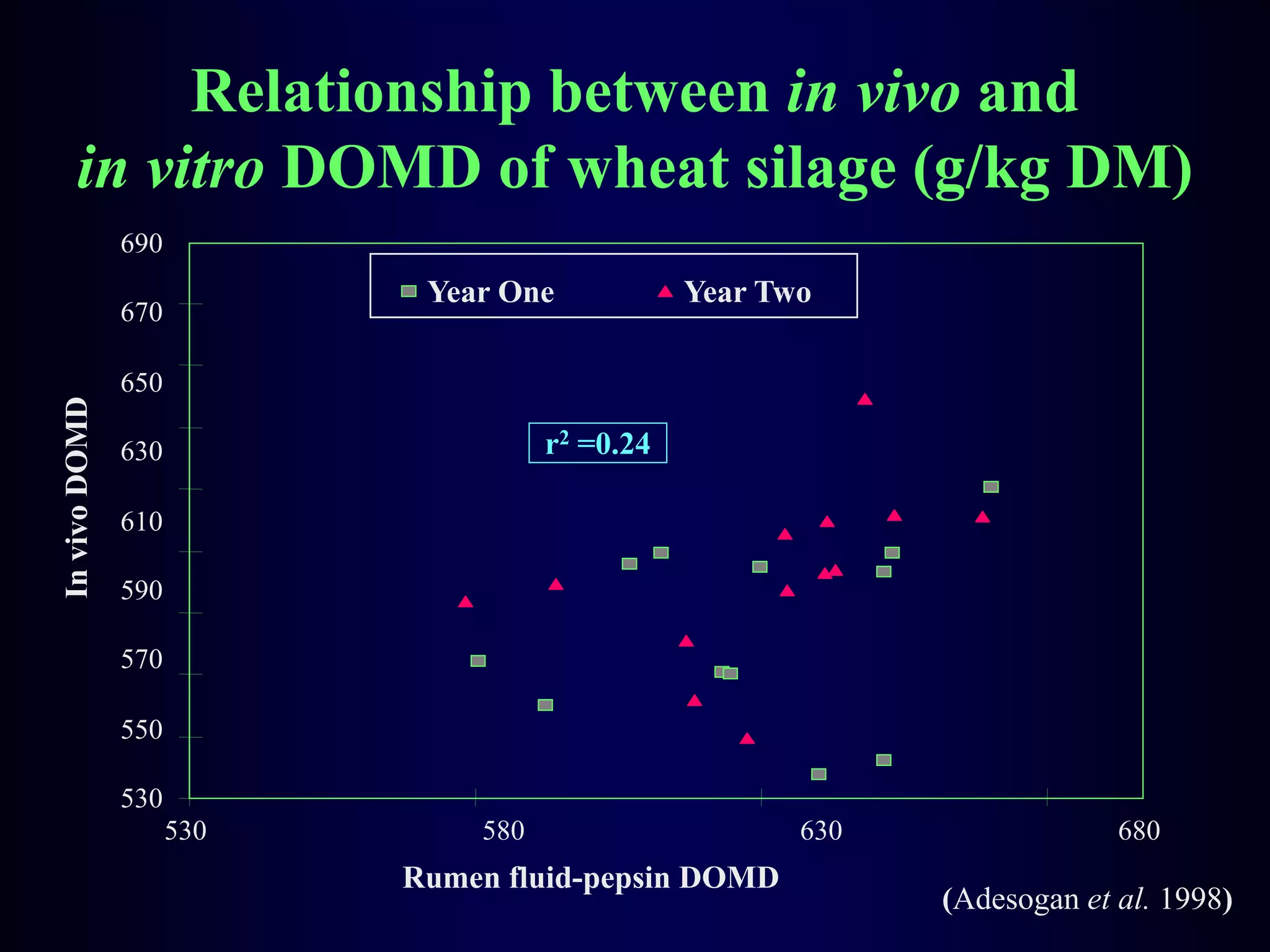
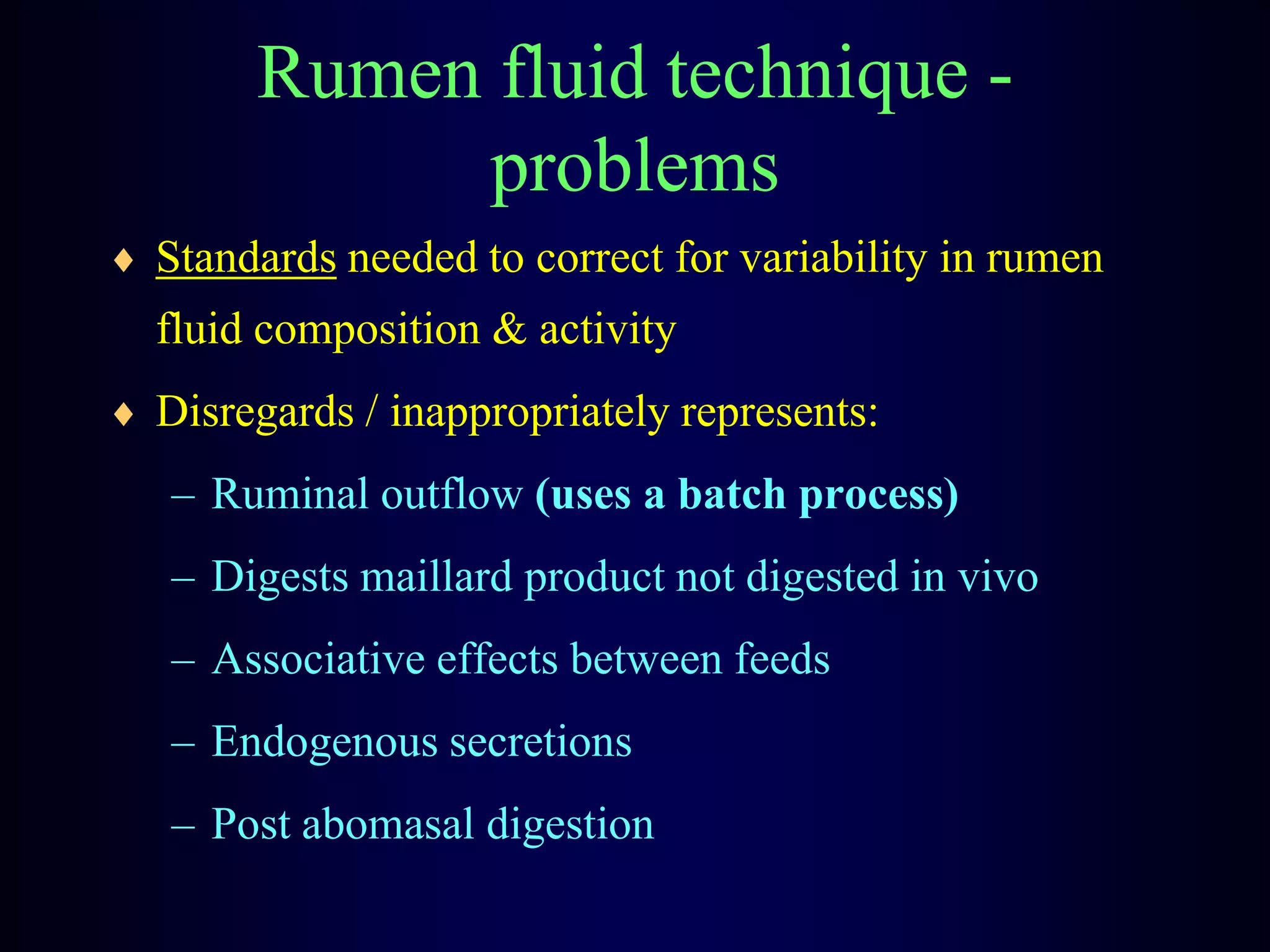
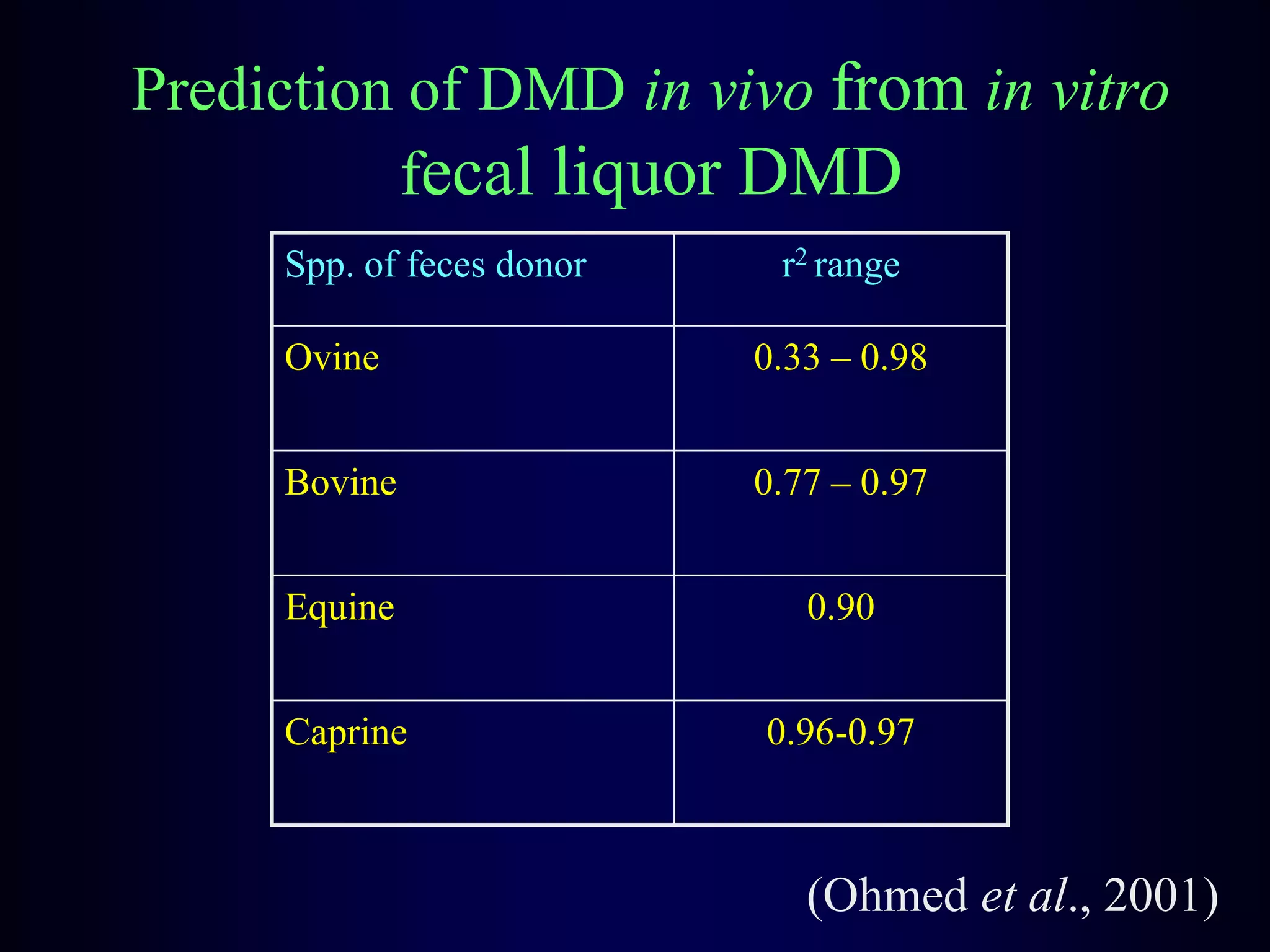
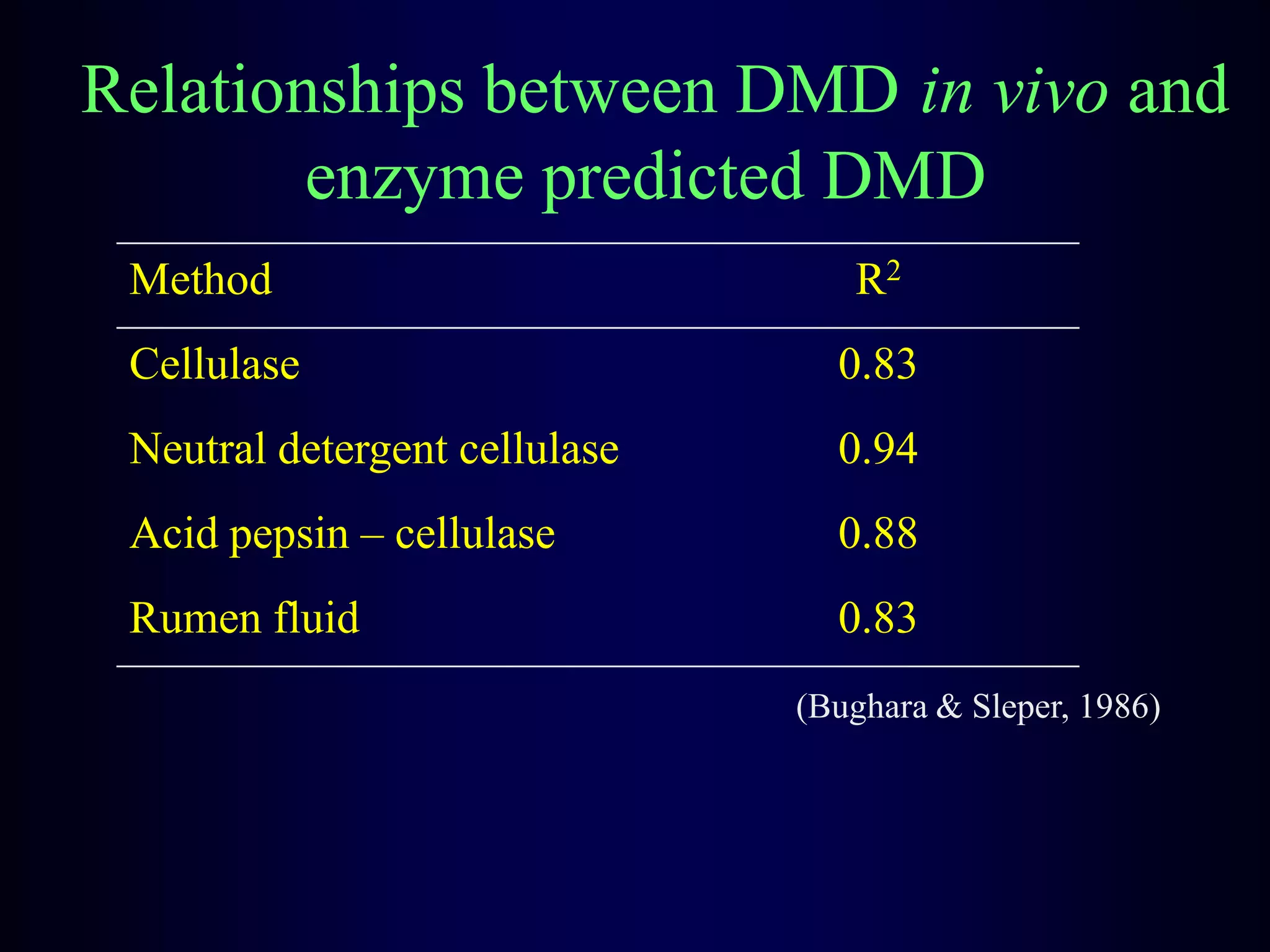
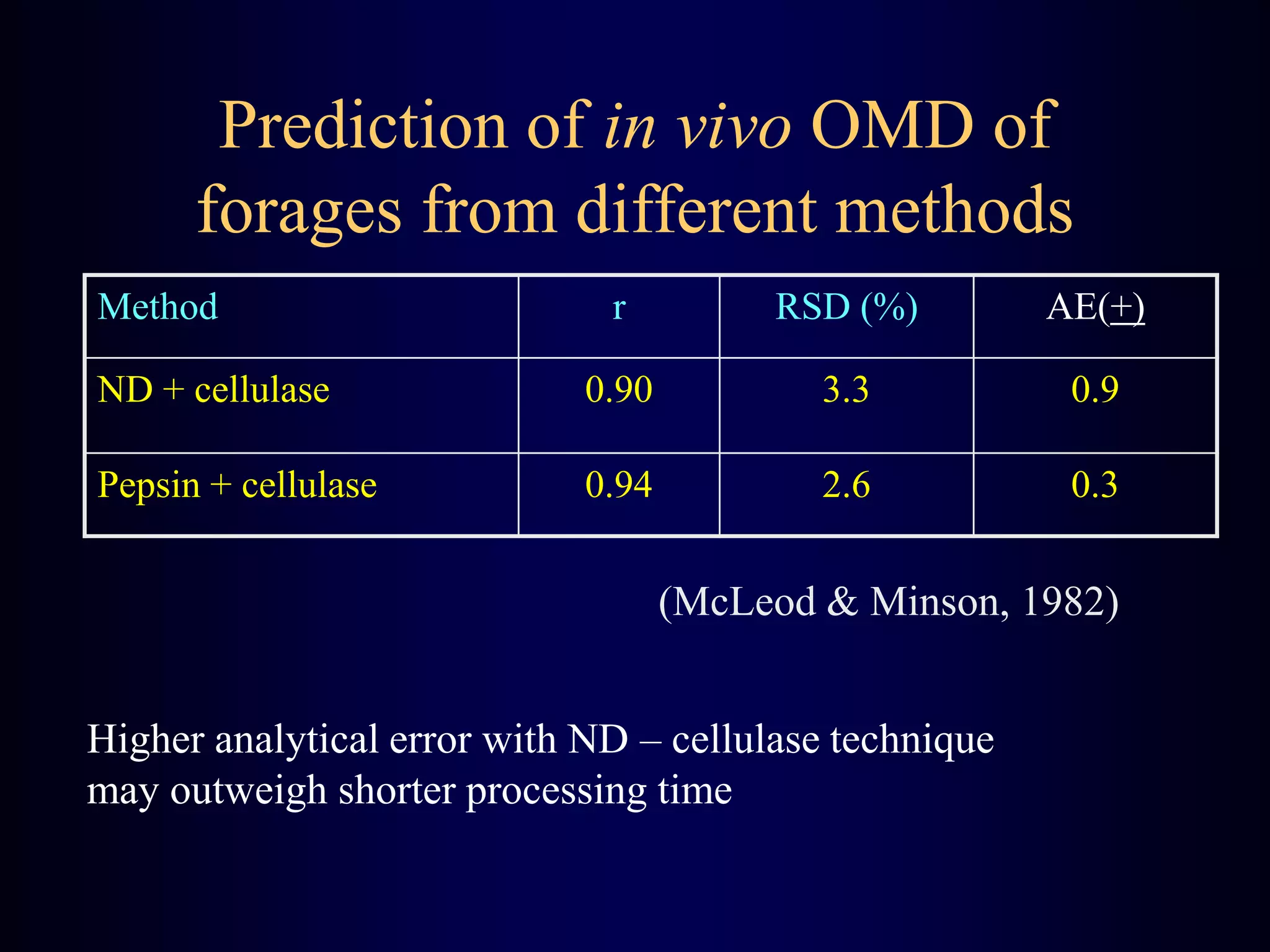
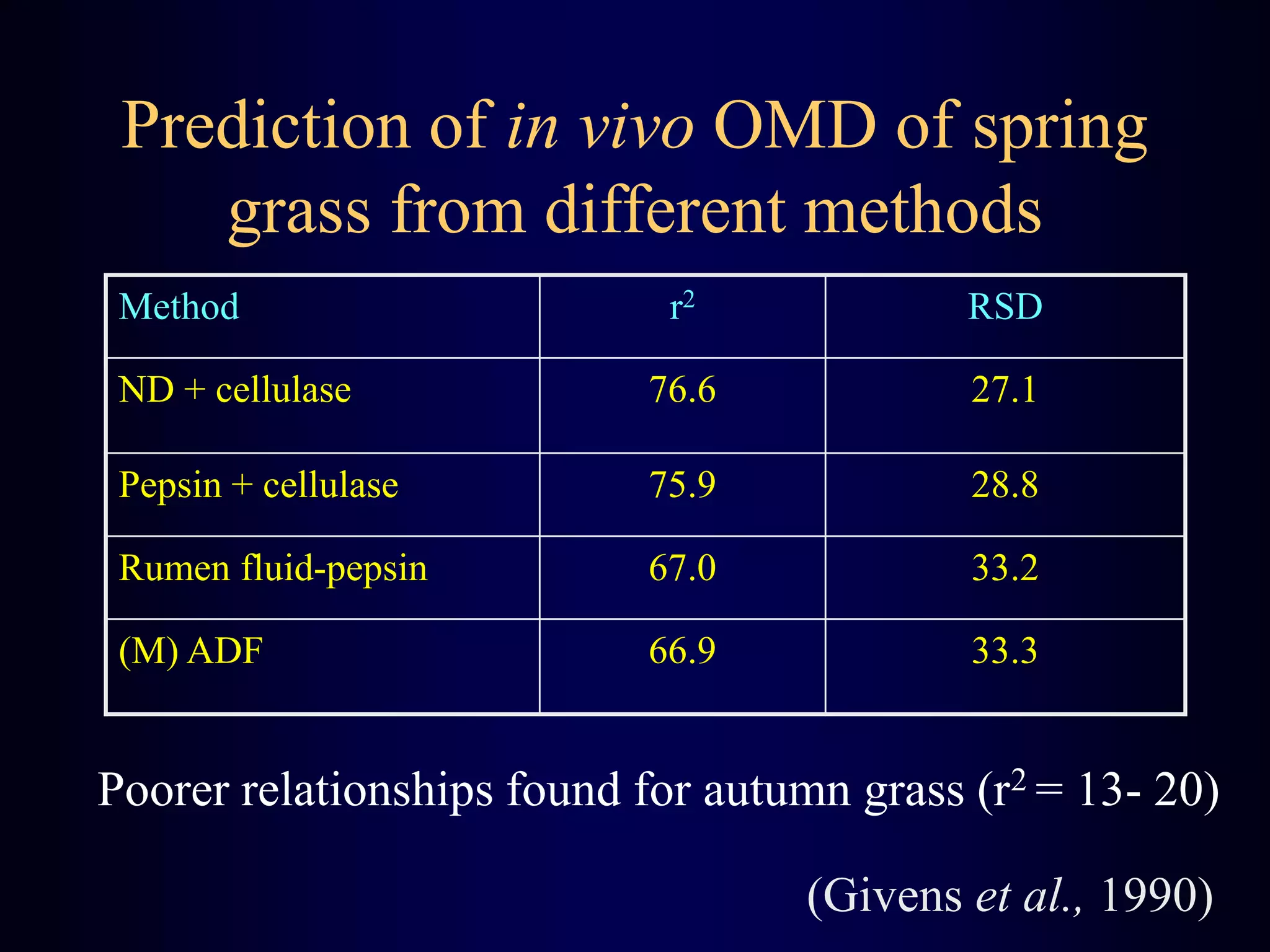
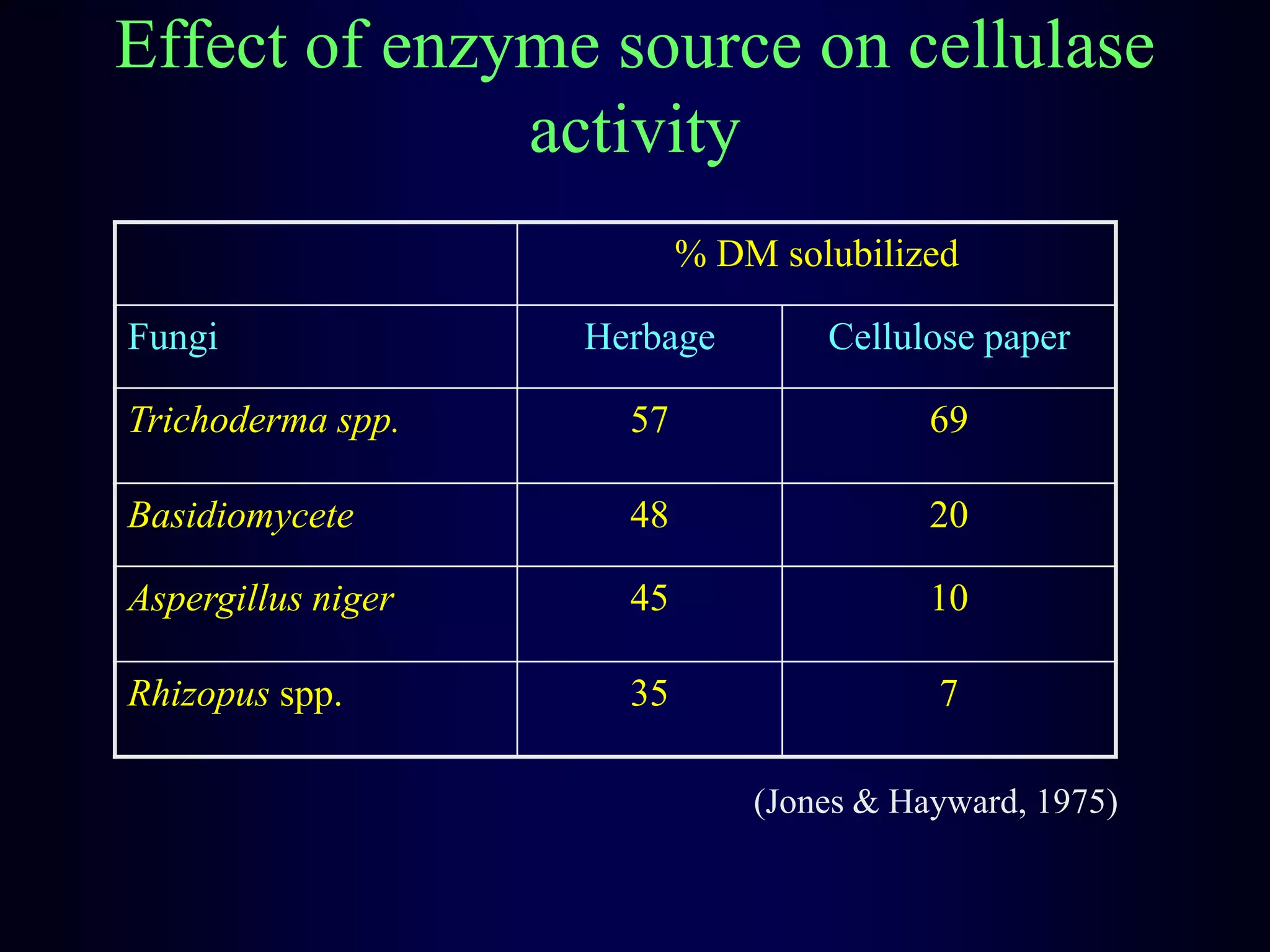
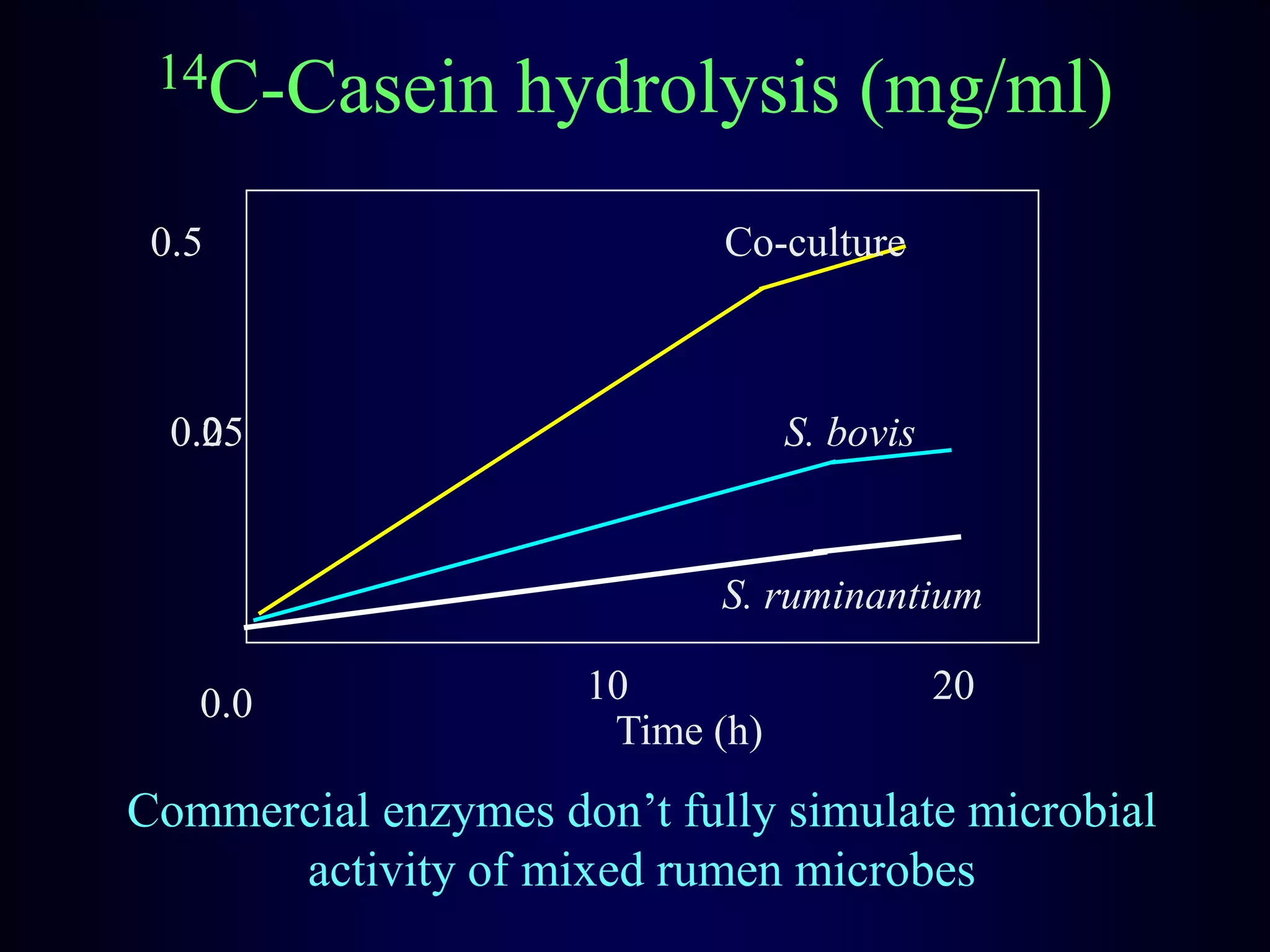
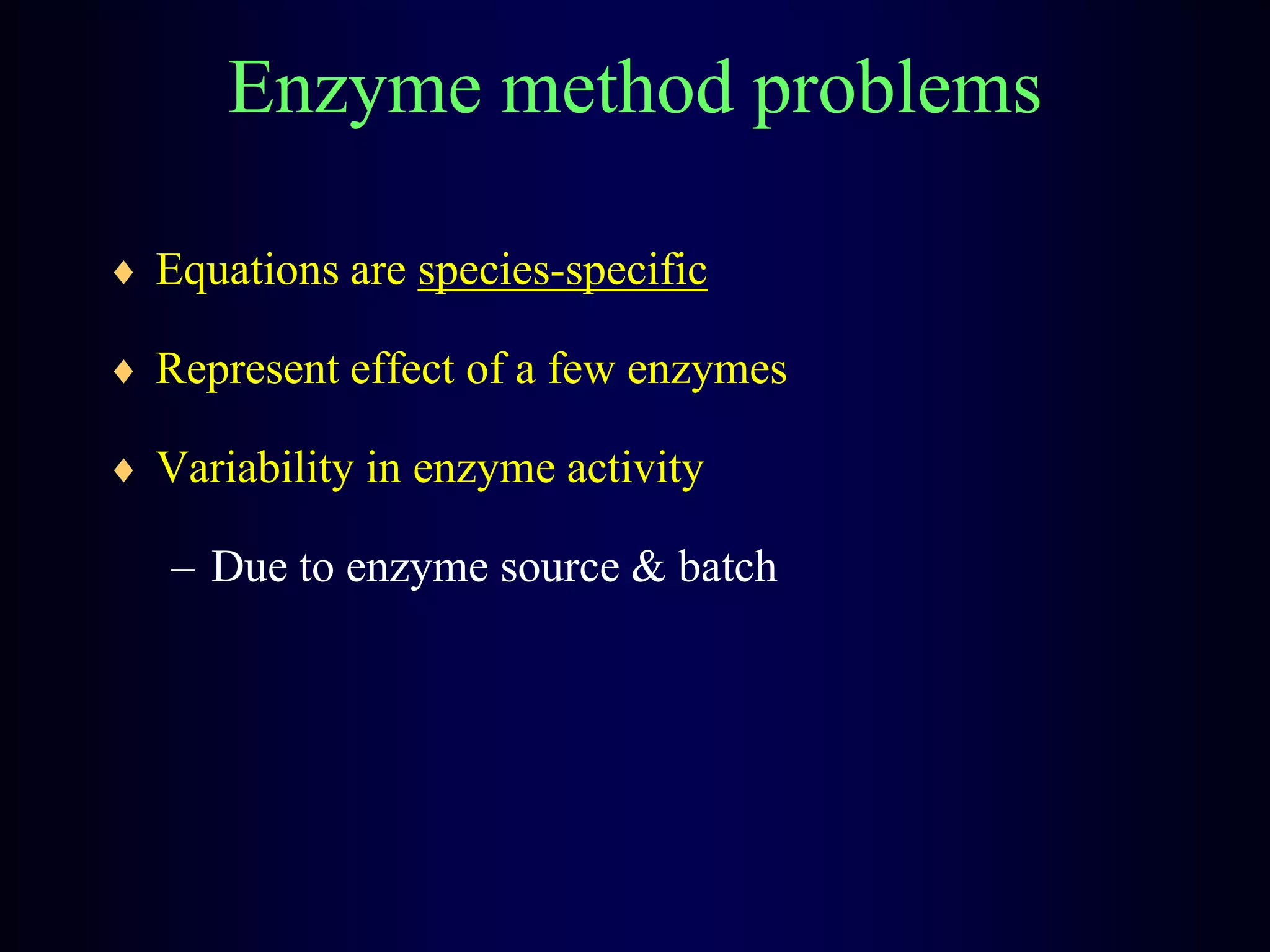
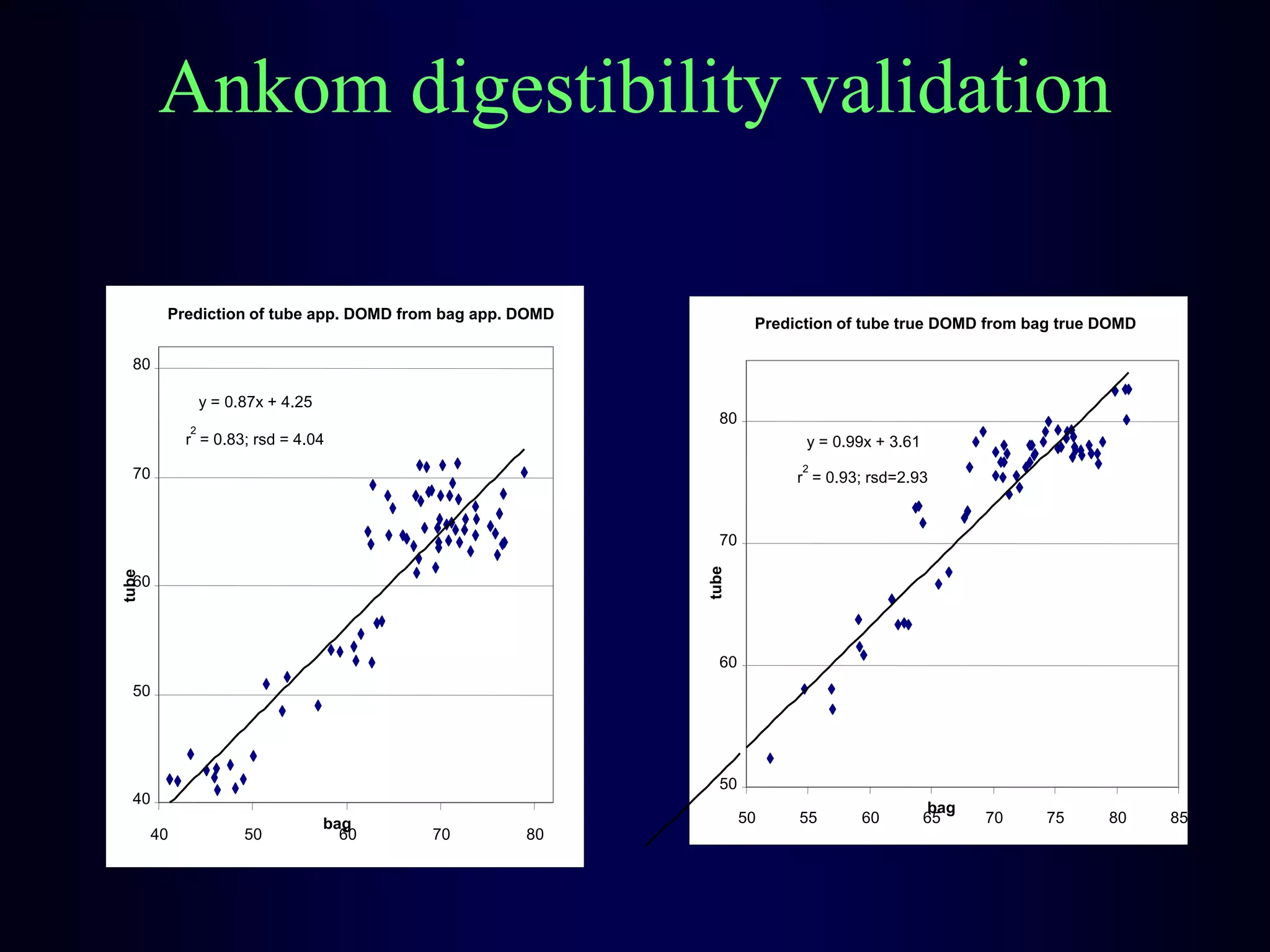
In vivo and in vitro methods are used to determine the digestibility of feeds for ruminants. True digestibility involves correcting for endogenous losses, while apparent digestibility does not. The rumen fluid-pepsin in vitro digestibility method provides accurate predictions of in vivo digestibility for most forages but has issues with variability in rumen fluid composition and activity. Cell-free enzyme methods address some limitations but do not fully represent the microbial activity in the rumen. Validation is needed to improve accuracy and consistency of in vitro digestibility methods.