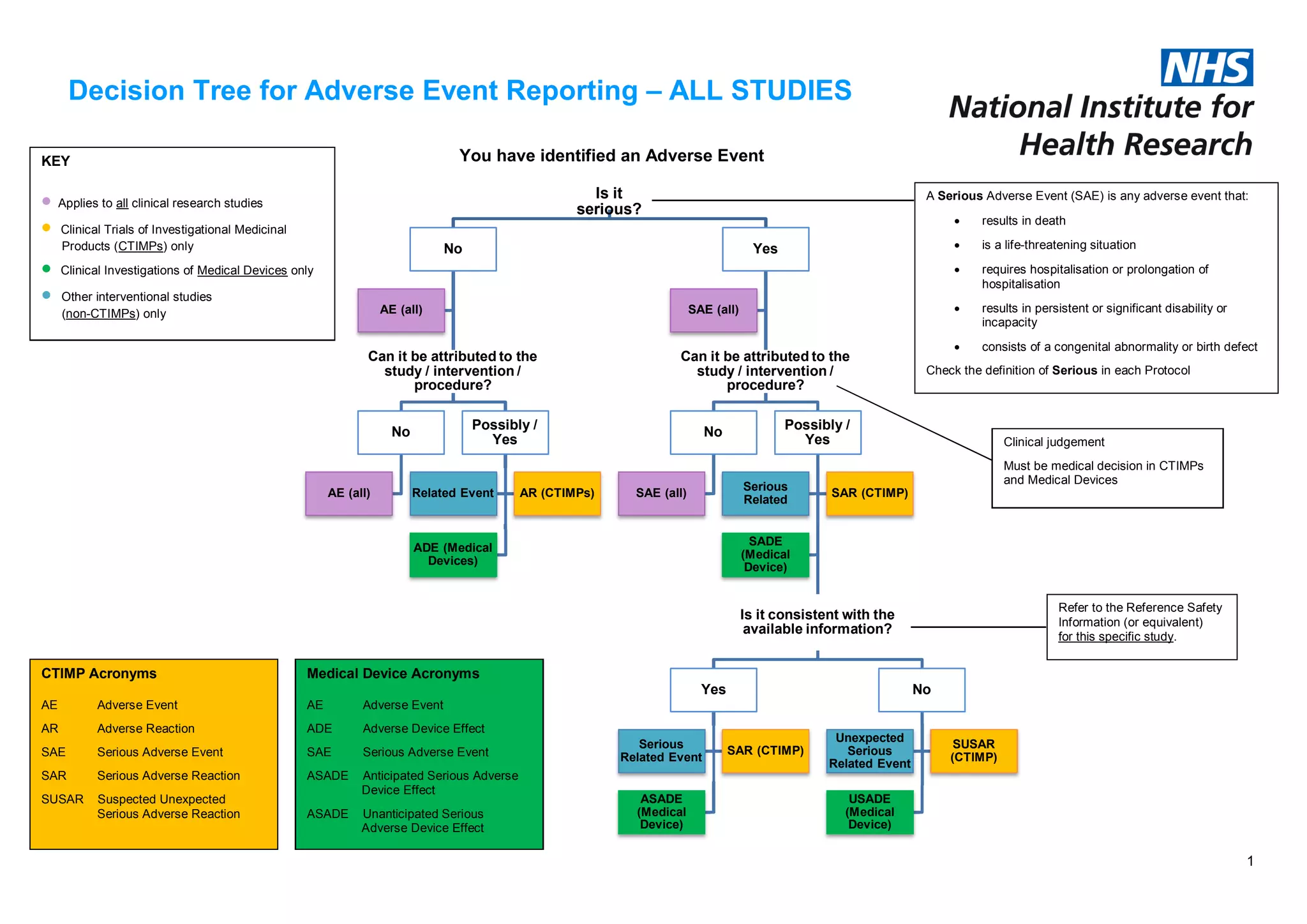
The document outlines a decision tree for reporting adverse events in clinical research studies, categorizing them based on their seriousness and relation to the study. It defines terms such as serious adverse event (SAE), adverse reaction (AR), and provides specific criteria for categorization in the context of clinical trials and medical devices. The decision tree applies to all studies and emphasizes the importance of clinical judgment in assessing adverse events.