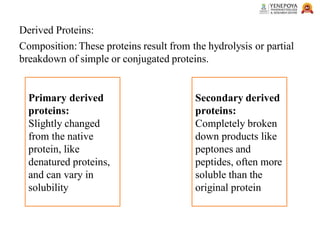
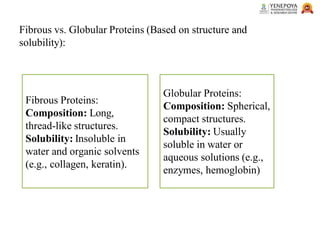
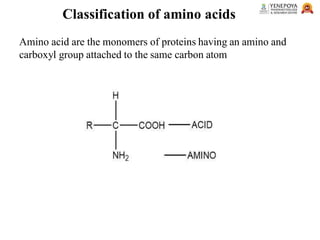
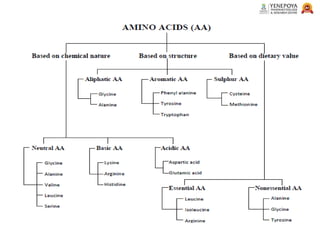
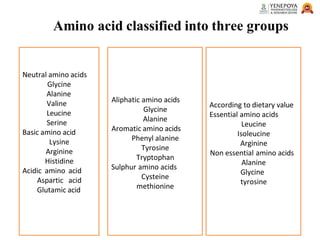
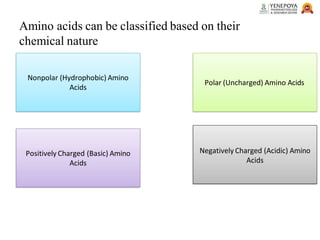
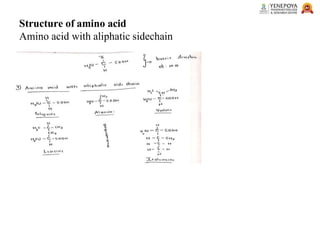
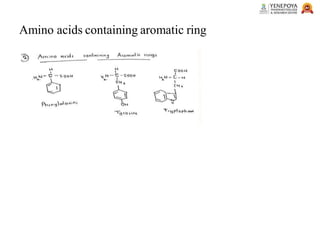
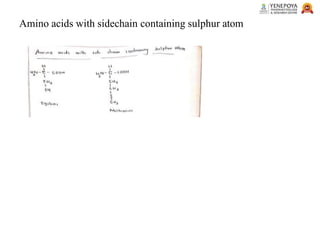
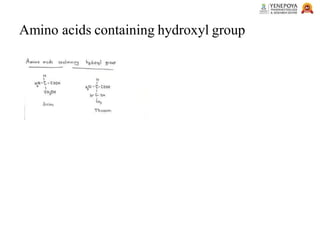
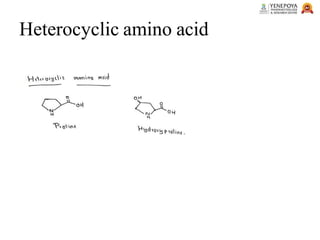
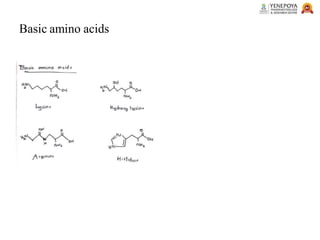
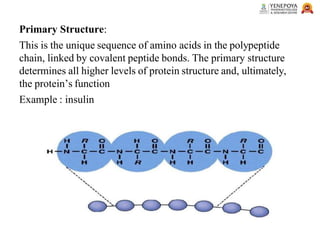
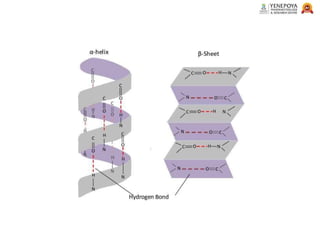
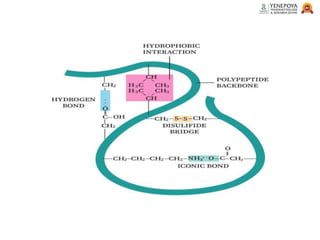
This presentation provides a comprehensive overview of proteins, covering their definition, classification, and structures, along with the classification of amino acids. It explains the biological roles of proteins and amino acids and highlights key diseases related to protein malnutrition such as Kwashiorkor, Marasmus, and Nutritional Edema. Ideal for pharmacy and life science students.