Clinical Trial Design and Artificial Intelligence | Pepgra.com
•
0 likes•148 views
Clinical trials take up the last half of the 10 – 15 year, 1.5 – 2.0 billion USD, cycle of development just for introducing a new drug within a market. 1. AI and its Evolution 2. AI in Clinical Trials To Continue Reading: https://bit.ly/2W01UDQ Contact Us: Website : https://bit.ly/33Fwsye Email us: sales.cro@pepgra.com Whatsapp: +91 9884350006
Report
Share
Report
Share
Download to read offline
Recommended
How Artificial Intelligence in Transforming Pharma

Artificial intelligence in Pharma refers to the use of automated algorithms to perform tasks which traditionally rely on human intelligence. Over the last five years, the use of artificial intelligence in the pharma and biotech industry has redefined how scientists develop new drugs, tackle disease, and more.
Given the growing importance of Artificial Intelligence for the pharma industry, we wanted to create a comprehensive report which helps every business leader understand the biggest breakthroughs in the biotech space which are assisted by the deployment of artificial intelligence technologies.
ARTIFICIAL INTELLIGENCE IN DRUG DISCOVERY "AN OVERVIEW OF AWARENESS"

ARTIFICIAL INTELLIGENT IN DRUG DISCOVERY:- AN OVERVIEW OF AWARENESS.
AI is showing the potential to be a faster and more efficient way to find and develop new drugs. A growing number of organizations and universities are focusing to minimize the complexities involved in the classical way of drug discovery by using AI computing to envisage which drug candidate are most likely to be effective treatments.
It is hard to measure the adoption of AI in drug discovery. Pharma and biotech companies tend to not publicly disclose competitive technology use.
While organizations are adopting the technology, there is significant untapped potential for those willing to be more aggressive. Which is depending on the realization of the potential with education and relevant success stories
Artificial intelligence and its applications in healthcare and pharmacy

Artificial Intelligence- Introduction, Scope, Problems of AI, Approaches to overcome them. Applications of AI Healthcare and Pharmacy.
Vaccine pharmacovigilance _ rajiv ahlawat

This presentation discuss about the importance, need and methods of vaccine Pharmacovigilance.
Good Clinical Practice and Pharmacovigilance

GCP: An international ethical and scientific quality standard for designing, conducting, recording and reporting clinical trials that involve the participation of human subjects.
PV: The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.
Recommended
How Artificial Intelligence in Transforming Pharma

Artificial intelligence in Pharma refers to the use of automated algorithms to perform tasks which traditionally rely on human intelligence. Over the last five years, the use of artificial intelligence in the pharma and biotech industry has redefined how scientists develop new drugs, tackle disease, and more.
Given the growing importance of Artificial Intelligence for the pharma industry, we wanted to create a comprehensive report which helps every business leader understand the biggest breakthroughs in the biotech space which are assisted by the deployment of artificial intelligence technologies.
ARTIFICIAL INTELLIGENCE IN DRUG DISCOVERY "AN OVERVIEW OF AWARENESS"

ARTIFICIAL INTELLIGENT IN DRUG DISCOVERY:- AN OVERVIEW OF AWARENESS.
AI is showing the potential to be a faster and more efficient way to find and develop new drugs. A growing number of organizations and universities are focusing to minimize the complexities involved in the classical way of drug discovery by using AI computing to envisage which drug candidate are most likely to be effective treatments.
It is hard to measure the adoption of AI in drug discovery. Pharma and biotech companies tend to not publicly disclose competitive technology use.
While organizations are adopting the technology, there is significant untapped potential for those willing to be more aggressive. Which is depending on the realization of the potential with education and relevant success stories
Artificial intelligence and its applications in healthcare and pharmacy

Artificial Intelligence- Introduction, Scope, Problems of AI, Approaches to overcome them. Applications of AI Healthcare and Pharmacy.
Vaccine pharmacovigilance _ rajiv ahlawat

This presentation discuss about the importance, need and methods of vaccine Pharmacovigilance.
Good Clinical Practice and Pharmacovigilance

GCP: An international ethical and scientific quality standard for designing, conducting, recording and reporting clinical trials that involve the participation of human subjects.
PV: The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.
various approaches to drug discovery

clinical and preclinical approaches to drug discovery.Here we mainly deals with preclinical approaches, ie. Pharmacological approach and toxicological approach
Drug development process

Pre-discovery
Understand the disease
Target Identification
Choose a molecule to target with a drug
Target Validation
Test the target and confirm its role in the disease
Drug Discovery
Find a promising molecule (a “lead compound”)
that could become a drug
Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel

Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel, April 20, 2021. Origin Story: Centralization Enables Decentralization. Analogous potential for centralization
leading to decentralization in clinical trials. Decentralization: Purpose and potential benefits, including resilience and
business continuity. Pre-Pandemic DCT Timeline: 17-year History Prior to COVID-19. Seasons of Decentralization in 2020. Spring of Continuity, Summer of Restarts, Fall of Commitment, Winter of Pathways to Scale. 79% of sponsors / CROs increasing DCT. 90% of participants experiencing change. 75% focus on going hybrid. 73% of Sites Will continue to use telemedicine beyond the pandemic. 76% have accelerated their DCT Strategies.Leading Implementation Strategy: Pairing DCT Toolkit to Study Needs. Identify the decentralized research methods and tools needed by the medicine portfolio. Ensure aligned SOPs & training, identify new partners, modify protocols/templates. Pair the “right” method/tool to each study
based upon diverse criteria. Barriers to Scaled Adoption of Decentralized Trials: Regulatory ambiguity, Global variability, Technology interop & data flow, Investigator & patient readiness, Endpoint limitations, Organization culture. Forecasts and Futures. Choice & Flexibility for Participants on a Visit-by-Visit Basis. Research Sites Empowered to Use Their Existing Technology. New Opportunities to Engage Treating Physicians Enables Research as a Care Option. Observational
“All-Comer” Studies and Platform Trials with DCT Bring Research to People.
INVESTIGATOR’S BROCHURE (IB)

The Investigator's Brochure (IB) is a comprehensive document summarizing the body of information about an investigational product (IB) obtained during a drug trial.
D&C Act 1940 Schedule Y - A Presentation by Akshay Anand

A Presentation about D&C Act 1940 - Schedule Y, Amendment 2005 by Akshay Anand, covering aspects of Rules and Guidelines to be followed in conduct of Clinical Trials in India. Presented in 2014.
Anatomical, therapeutic and chemical classification of drugs.pptx

Anatomical, therapeutic and chemical classification of drugs
Pharmacovigilance: An umbrella word for DRug safety.

In this PPT All type of information regarding pharmacovigilance is to be given. i hope this will give your answers regarding p'covigilance.
List out the challenges of ml ai for delivering clinical impact - Pubrica

Pubrica explores the main challenges and limitations of AI in healthcare and considers the steps required to translate these potentially transformative technologies from research to clinical practice.
Continue Reading: https://bit.ly/3o4hjPT
Reference: https://pubrica.com/services/research-services/biostatistics-and-statistical-programming-services/
Why Pubrica?
When you order our services, Plagiarism free|on Time|outstanding customer support|Unlimited Revisions support|High-quality Subject Matter Experts.
Contact us :
Web: https://pubrica.com/
Blog: https://pubrica.com/academy/
Email: sales@pubrica.com
WhatsApp : +91 9884350006
United Kingdom: +44- 74248 10299
International Approaches to Health Information Technology Safety

With the introduction of new technologies, there are opportunities to introduce new types of medical errors (i.e. technology-induced errors). Technology-induced errors arise from interactions between citizens, patients and health professionals and the technologies they use to provide health information and health care (Borycki & Kushniruk, 2008).
More Related Content
What's hot
various approaches to drug discovery

clinical and preclinical approaches to drug discovery.Here we mainly deals with preclinical approaches, ie. Pharmacological approach and toxicological approach
Drug development process

Pre-discovery
Understand the disease
Target Identification
Choose a molecule to target with a drug
Target Validation
Test the target and confirm its role in the disease
Drug Discovery
Find a promising molecule (a “lead compound”)
that could become a drug
Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel

Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel, April 20, 2021. Origin Story: Centralization Enables Decentralization. Analogous potential for centralization
leading to decentralization in clinical trials. Decentralization: Purpose and potential benefits, including resilience and
business continuity. Pre-Pandemic DCT Timeline: 17-year History Prior to COVID-19. Seasons of Decentralization in 2020. Spring of Continuity, Summer of Restarts, Fall of Commitment, Winter of Pathways to Scale. 79% of sponsors / CROs increasing DCT. 90% of participants experiencing change. 75% focus on going hybrid. 73% of Sites Will continue to use telemedicine beyond the pandemic. 76% have accelerated their DCT Strategies.Leading Implementation Strategy: Pairing DCT Toolkit to Study Needs. Identify the decentralized research methods and tools needed by the medicine portfolio. Ensure aligned SOPs & training, identify new partners, modify protocols/templates. Pair the “right” method/tool to each study
based upon diverse criteria. Barriers to Scaled Adoption of Decentralized Trials: Regulatory ambiguity, Global variability, Technology interop & data flow, Investigator & patient readiness, Endpoint limitations, Organization culture. Forecasts and Futures. Choice & Flexibility for Participants on a Visit-by-Visit Basis. Research Sites Empowered to Use Their Existing Technology. New Opportunities to Engage Treating Physicians Enables Research as a Care Option. Observational
“All-Comer” Studies and Platform Trials with DCT Bring Research to People.
INVESTIGATOR’S BROCHURE (IB)

The Investigator's Brochure (IB) is a comprehensive document summarizing the body of information about an investigational product (IB) obtained during a drug trial.
D&C Act 1940 Schedule Y - A Presentation by Akshay Anand

A Presentation about D&C Act 1940 - Schedule Y, Amendment 2005 by Akshay Anand, covering aspects of Rules and Guidelines to be followed in conduct of Clinical Trials in India. Presented in 2014.
Anatomical, therapeutic and chemical classification of drugs.pptx

Anatomical, therapeutic and chemical classification of drugs
Pharmacovigilance: An umbrella word for DRug safety.

In this PPT All type of information regarding pharmacovigilance is to be given. i hope this will give your answers regarding p'covigilance.
What's hot (20)
Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel

Decentralized Clinical Trials, presentaiton by Craig Lipset for mHealth Israel
D&C Act 1940 Schedule Y - A Presentation by Akshay Anand

D&C Act 1940 Schedule Y - A Presentation by Akshay Anand
Anatomical, therapeutic and chemical classification of drugs.pptx

Anatomical, therapeutic and chemical classification of drugs.pptx
Pharmacovigilance: An umbrella word for DRug safety.

Pharmacovigilance: An umbrella word for DRug safety.
Similar to Clinical Trial Design and Artificial Intelligence | Pepgra.com
List out the challenges of ml ai for delivering clinical impact - Pubrica

Pubrica explores the main challenges and limitations of AI in healthcare and considers the steps required to translate these potentially transformative technologies from research to clinical practice.
Continue Reading: https://bit.ly/3o4hjPT
Reference: https://pubrica.com/services/research-services/biostatistics-and-statistical-programming-services/
Why Pubrica?
When you order our services, Plagiarism free|on Time|outstanding customer support|Unlimited Revisions support|High-quality Subject Matter Experts.
Contact us :
Web: https://pubrica.com/
Blog: https://pubrica.com/academy/
Email: sales@pubrica.com
WhatsApp : +91 9884350006
United Kingdom: +44- 74248 10299
International Approaches to Health Information Technology Safety

With the introduction of new technologies, there are opportunities to introduce new types of medical errors (i.e. technology-induced errors). Technology-induced errors arise from interactions between citizens, patients and health professionals and the technologies they use to provide health information and health care (Borycki & Kushniruk, 2008).
AI in Healthcare: From Hype to Impact (updated)

The primary goal of this workshop is to help health professionals gain a critical understanding of the various types of AI technologies available so they can make wise decisions and invest AI for healthcare improvement.
1Milestone 1Deanna BuchananSouthern New Hampsh.docx

1Milestone 1Deanna BuchananSouthern New Hampsh.docx
1Milestone 1Deanna BuchananSouthern New Hampsh

1
Milestone 1
Deanna Buchanan
Southern New Hampshire University
HIM-500-Q1513
Milestone 1
In the field of health informatics, particular historical events help inform the management of health information:
1. The 1950s saw the early development of health informatics through cybernetics and information processing. This entailed professionals from various fields, such as clinical documentation and epidemiology.
2. The 1960 to 2000 period saw the evolution of data analysis and computing. Health information management was facilitated through the development of electronic medical records systems. Such systems are vital for health information management since they provide real-time patient-centered records to authorized users (Collen & Ball, 2018).
3. There is the period from 2000 to the present where stakeholders have moved to digitize healthcare processes such as information sharing, record keeping, and care coordination.
Guidelines
There are several guidelines for technology use that Feather fall could implement in health information management. For starters, the medical staff should get the relevant training required to utilize the technology to facilitate the effective acquisition, analysis, and protection of patient information. Training ensures they can tackle any challenges they may encounter to collect accurate data and analyze it in a way that benefits patients. Additionally, the medical staff needs to ensure that the devices they use are beyond the reach of unauthorized individuals. This is crucial in promoting patient confidentiality/privacy and securing pertinent data does not get into the wrong hands (Ozair et al., 2018). Finally, medical practitioners should provide feedback about their experiences to ensure that the technology they use can be improved in the future.
Standard Technologies
There are various standard technologies used in health information management. For starters, concerning record keeping, some of the traditional EHR technologies include Epic Systems and Meditech. Many institutions use these two systems due to their departmental functionality and extensive usability. RingCentral Video is a standard technology for videoconferencing that facilitates open communication and interactive communications among healthcare providers. Different practitioners can share information and work together in real-time to facilitate effective patient care. Finally, there is the use of Vendor-Neutral Archives (VNAs) and Picture Archiving and Communication Systems (PACS) when it comes to processing and storing the medical images of patients (Sirota-Cohen et al., 2019).
How Roles at Feather fall Interact with Technology
The pertinent roles at Feather fall would interact with technology through a simple but effective communication system that ensures all users can get the most out of the health management technologies on offer. Currently, the staff members have poor training and no means of effe ...
Impact of Artificial Intelligence in the Pharmaceutical World A Review

The pharmaceutical industry stands to be transformed by Artificial Intelligence AI , particularly in areas such as drug discovery, clinical trials, and personalized medicine. However, there are several obstacles to implementing AI in this industry, including limited familiarity with the technology, inadequate IT infrastructure, and the difficulty of extracting valuable data from patients records. One specific application of AI in the pharmaceutical field involves the development of small peptides with antimicrobial properties, which can serve as novel antibiotics to combat superbugs that are resistant to multiple drugs. AI can assist in determining the effectiveness and potency of these peptides, facilitating the development of powerful antibiotics. Despite these challenges, AI holds tremendous potential in the pharmaceutical industry, enabling accelerated innovation, time and cost savings, and ultimately, saving lives. In conclusion, although there are limitations to adopting AI in pharma, there are numerous promising future prospects that could revolutionize the industry and enhance patient outcomes. Shaikh Sameer Salim | Manoj Kumar "Impact of Artificial Intelligence in the Pharmaceutical World- A Review" Published in International Journal of Trend in Scientific Research and Development (ijtsrd), ISSN: 2456-6470, Volume-7 | Issue-3 , June 2023, URL: https://www.ijtsrd.com.com/papers/ijtsrd57564.pdf Paper URL: https://www.ijtsrd.com.com/computer-science/artificial-intelligence/57564/impact-of-artificial-intelligence-in-the-pharmaceutical-world-a-review/shaikh-sameer-salim
IoE in Clinical Trials

This article is taken from International Clinical Trials November 2017, pages 20-24. © Samedan Ltd
Generative AI in Healthcare Market - Copy - Copy.pptx

Generative AI holds significant promise in healthcare, there are also challenges related to data privacy, model interpretability, and regulatory compliance that need to be addressed. Ethical considerations and thorough validation processes are crucial to ensure the responsible and safe application of generative AI techniques in healthcare.
Disruptors in the Medical Imaging Industry

An overview of the Disruptors in the Medical Imaging Market. This free webinar will also give you more insight on the various factors that influence the market. We touch on results from a survey of a survey of 147 radiologists highlight the importance of reimbursement changes –both “appropriateness” measures and value-based medicine – as the most significant factors that will impact the imaging market.
Him500 Milestone 3Precious Teasley Southern New 

Him500 Milestone 3
Precious Teasley
Southern New Hampshire University
Him500
Professor Jon McKeeby
February 20,2022
Him500 Milestone 3
Organization Needs
Government laws and regulations have been broken because of Featherfall Medical Center's outdated technology. Staffs are not only out of date in terms of skills, but the technology itself is also outdated. Discrepancies in government regulations, operational problems, and ethical dilemmas stemming from poor technology implementation have all cost the organization money. Featherfall's technological needs have been whittled down to Alert (Admission, Discharge, and Transfer ADT') and Intel, two eligible vendors (SOA Expressway for Healthcare). These systems need to satisfy three key goals: to meet personnel demands, protect the integrity of healthcare, and meet government standards. Concerns about the expense of implementing and maintaining a new system are high because Featherfall contains consequences for earlier infractions. Choosing a new computer system for Featherfall Medical Center is the right decision. Due to legislative rules, the medical center's obsolete system has severely impacted the organization's finances. In addition, they have problems maintaining the accuracy of their medical records. Some sectors suffer from a lack of training and clear communication channels. The medical center's new system must meet HIPAA compliance rules, communicate effectively amongst itself, be user-friendly for the personnel, and be under governmental regulations to be accepted.
Technology System Recommendations
In my opinion, Intel is the best new technology for Featherfall Medical Center (SOA Expressway for Healthcare). For Featherfall Medical Center, I feel Intel is an attractive choice because the system is simple to use and can be rapidly adopted into regular tasks. The technology will also allow for more outstanding communication between the staff. It will be easier to manage patient care with Intel since the system will produce discharge and transfer lists. In addition, the system can produce records of patients by their doctors and patients by their departments. The system is password-protected and features multiple levels of security. HIPAA compliance has not yet been achieved, but UHDDS is in place and working as intended. The next release will meet HIPAA regulations (Durcevic, 2019). Because of Intel's size and wealth of knowledge, you can be assured that your health information is in good hands.
Financial Resources
Intel is more expensive than the Alert (ADT) system, but it has the greater experience. As a company around for 30 years, Intel has 364 medical systems in use. There was a total cost of $2,028,000 for Intel and $1,587,000 for Alert. Compared to Alert, Intel was $441,000 more expensive (SNHU, 2019). Featherfall Medical Center will benefit from Intel's knowledge and resources as a larger firm. System costs more, but it will help with compliance, ethics, and govern ...
Artificial intelligence in healthcare market global trends, market share, ind...

Global Artificial Intelligence in Healthcare Market is estimated to value over USD 37 billion by 2029 end and is expected to register a CAGR of over 50% during the forecast period 2019 to 2029.
The artificial intelligence (AI) is capable of improving patient outcomes by accurately identifying the source cause of the disease, this is positively influencing the market growth.
AssignmentThe rough draft for your course project is now due..docx

Assignment
The rough draft for your course project is now due.
Your rough draft should include a cover page, 5 - 7 pages of writing in the body of the paper, and a reference page. The paper should demonstrate a strong overview of the use of technology in your selected area.
Your research should include at least six references. The paper must use in-text citations and references in APA format. You can find more information on APA format in the Online Library, which is accessible through the Resources tab.
Be sure to proofread your paper one more time. Also, make a checklist of the requirements found in
Module 02 Course Project - Introduction.
Pervious assignment*
Technology has changed the way healthcare services are provided
in this day and age. New tools and ways of doing things have been introduced enabling doctors to be more efficient when doing surgery. Doctors do not even need to be in the same locations as the patient to do surgery or prescribe medication. Knowledge from all over the world can be accessed with the touch of a button this has improved the provision of medical services tremendously. This has benefited many people all over the world and made healthcare delivery easier (Lamba, 2011).
Some of the equipment that has been brought about by technology advancement include; 3D echocardiogram and CT reconstruction. These two have made surgery more efficient by improving imaging capabilities which enable surgeons to operate without hindrances on the human body (Sabik, MD, 2014). Healthcare services have improved too, by the introduction of robotic surgery and teleconferencing. The latter has enabled doctors to provide knowledge and expertise far and wide irrespective of their physical location. They can monitor, track and communicate with their patients to help them keeping tabs on their ailments (Raza, et al., 2014). Robotic surgery helps doctors conduct surgery remotely with the use of a machine that can multitask and overcome some of the limitations a human doctor may have. This improves the likelihood of an operation to be successful which benefits both the physician and the patient.
Use of these new technologies is compulsory as not only have they made surgery more efficient but also tackled some challenges that were considered impossible in the past such as organ replacement and valve replacement in the aorta (Lamba, 2011). In addition to this technology has changed the way surgery is done by finding more effective and efficient ways of carrying out complicated procedures through extensive research. It is not a surprise that there are now less invasive operation procedures that enable doctors work and stitch you up in no time leaving no scars.
References
Lamba, P. (2011). Teleconferencing in Medical Education: A Useful Too.
Australasian Medical Journal,, 4
(8), 442-447.
Raza, S., Sabik, F. J., Masabni, K., Ainkaran, P., Lytle, W. B., & Blackstone, H. E. (2014). Surgical revascularization techniques that minimize surgi ...
Similar to Clinical Trial Design and Artificial Intelligence | Pepgra.com (20)
List out the challenges of ml ai for delivering clinical impact - Pubrica

List out the challenges of ml ai for delivering clinical impact - Pubrica
International Approaches to Health Information Technology Safety

International Approaches to Health Information Technology Safety
1Milestone 1Deanna BuchananSouthern New Hampsh.docx

1Milestone 1Deanna BuchananSouthern New Hampsh.docx
Impact of Artificial Intelligence in the Pharmaceutical World A Review

Impact of Artificial Intelligence in the Pharmaceutical World A Review
CA499 Impact of Technology in Nursing Practice Research.pdf

CA499 Impact of Technology in Nursing Practice Research.pdf
Generative AI in Healthcare Market - Copy - Copy.pptx

Generative AI in Healthcare Market - Copy - Copy.pptx
Artificial intelligence in healthcare market global trends, market share, ind...

Artificial intelligence in healthcare market global trends, market share, ind...
AssignmentThe rough draft for your course project is now due..docx

AssignmentThe rough draft for your course project is now due..docx
More from PEPGRA Healthcare
Clinical Research Organization Services | Contract Research Company - Pepgra

Pepgra is a global contract research organization and drug development services company. It provides various phases of clinical research trials services to pharmaceutical and biotechnology companies to help reduce the time and costs associated with drug development.
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
India: +91 9884350006
United Kingdom: +44- 74248 10299
Different Stages of Medical Device Development and Drug Development: PepgraDi...

The difference between medical device product development and pharmaceuticals that are supposed to be launched are based on industry composition where above 80% small and medium-sized companies require medical devices and large multinational organizations seek new medicines. Pepgra gives you the different stages of Medical Device Development and Drug Development, some are:
1. Lead discovery optimization
2. Pre-clinical Research
3.. Clinical Research
4. Post- Market safety monitoring
Continue Reading: https://bit.ly/3ryCQC4
Youtube: https://www.youtube.com/watch?v=cYz_BOArGhA
If you need any further information, then please contact via
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
Top 5 tips for managing risks in your clinical studies - Pepgra

Fronting ever-increasing costs of running a clinical trial, sponsors must guarantee they are correctly directing their financial plan and resolving the highest risk areas while preserving patient safety and data reliability in Patient recruitment for clinical trials. In this blog, Pepgra provides five tips for significant risk levels in clinical studies like:
1. Outlining your levels of risks
2. Evaluating and categorizing risk
3. Concentrating on essential areas of risk
4. Observing and controlling risks
5. Estimating the efficiency of risk management
Read More: http://bit.ly/3bb4j6h
Youtube: https://youtu.be/EGH6WDsqSSw
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
India: +91 9884350006
United Kingdom: +44- 74248 10299
Role of Biostatistician and Biostatistical Programming in Epidemiological Stu...

Pepgra experts provide regulatory biostatistics and epidemiology statistical programming support to all phases of clinical trial process development and commercialization. Our Epidemiological statistical services is are located globally & trained in current methods and standards to support the successful execution of your projects.
Continue Reading: http://bit.ly/2OBq9EZ
Youtube: https://youtu.be/2NORssElgFg
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
India: +91 9884350006
United Kingdom: +44- 74248 10299
EMA Guidelines for Clinical Trial Management - Pepgra Healthcare

The European Medicines Agency (EMA) relies on the results of clinical trials carried out by pharmaceutical companies to reach its opinions on the authorisation of medicines. EMA guidelines for clinical trial regulations adopted in 2014 aim to make it easier for the clinical trials companies while empowering participants through transparency.
Continue Reading : http://bit.ly/36LJZa7
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
Side effects of drugs products, medical devices & drugs healthcare data...

Rapid growth in the use of medical devices in health-care sectors has been enabled by technological advancements. Prescription drugs and medical devices can come with dangerous side effects and complications. A side effect will become severe, so it is essential to contact your doctor or pharmacy specialist if you face ant difficulties. In this blog, Pepgra lists the reporting of side effects and its necessities.
Read More: http://bit.ly/3j0wisP
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
Guidance for Biomarkers into Early Phase Clinical Research Purposes | Healthc...

In many health data analytics companies, Biomarkers are playing increasingly critical roles in the development of new drugs in early-phase trials. This blog is meant to introduce clinical investigators to the fundamentals of choosing a biomarker test for use in an early phase trial. Some steps to consider are briefly outlined including defining the role of the biomarker in the early phase trial are:
Errors in Measurement, Bigotry, Astounding, Price and Acceptability.
Continue Reading: http://bit.ly/38TbC2H
Contact us:
UK: +44-1143520021
US/Canada: +1-972-502-9262
India: +91-9884350006
Email id: sales.cro@pepgra.com
Website: www.pepgra.com
Recent trends in genomic biomarkers pepgra healthcare

Cardiovascular disease is a significant health concern worldwide despite having many genomics developments providing valuable new candidates for better biomarkers and novel therapeutic targets. The main integration of new technologies promises the discovery and validation of better biomarkers of the presence of cardio disease, its progression, and the response to treatment in this blog. Some of the features are:
1. Analyzing the Gene expression
2. Genome-wide association studies
3. Linkage analysis
4. Wrapping up...
Continue Reading: http://bit.ly/3bqq3Np
Contact us:
UK: +44-1143520021
US/Canada: +1-972-502-9262
India: +91-9884350006
Email id: sales.cro@pepgra.com
Website: www.pepgra.com
Recent Trends in Genomic Biomarkers - Pepgra Healthcare

Cardiovascular disease is a significant health concern worldwide despite having many genomics developments providing valuable new candidates for better biomarkers and novel therapeutic targets. The main integration of new technologies promises the discovery and validation of better biomarkers of the presence of cardio disease, its progression, and the response to treatment in this blog. Some of the features are:
1. Analyzing the Gene expression
2. Genome-wide association studies
3. Linkage analysis
4. Wrapping up...
Continue Reading: http://bit.ly/3bqq3Np
Contact us:
UK: +44-1143520021
US/Canada: +1-972-502-9262
India: +91-9884350006
Email id: sales.cro@pepgra.com
Website: www.pepgra.com
Guidelines on Virtuous Pharmacovigilance Practices | Pepgra Healthcare

Pepgra explains the general guidelines of pharmacovigilance for all clinical research organization. Medical-related problems for the Clinical trial monitoring services have a considerable impact on the pharmacovigilance process as it detects monitors and analyze the medical activities. Some general guidelines to promote the regulations in health care sectors are given below:
• Planning of quality
• Adherence to quality
• Quality assurance and control
• Improvements in quality
• Documenting the legal requirements
• Protecting humans from the adverse effects
• Building safe and practical applications
Continue Reading: http://bit.ly/2WosJSS
Contact Us:
Website: https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
Challenges and-opportunities-in-software-driven-medical-sciences

SaMD or Software as a Medical Device can be described as a software constructed to be used in medical devices. These softwares can be run on different operating systems and virtual platforms.
1. The basic programming model of a SaMD is given below.
2. Different softwares are used for medical purposes, and they include the following:
To continue Reading : https://bit.ly/31ItRVc
Contact Us:
Website : https://bit.ly/2BvO06b
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
Clinical trail-designchallenges-in-the-study-design-conduct-and-analysis-of-r...

•The major steps in conducting a clinical trial study are study design, study conduct, data analysis and reporting of the findings.
•Randomized clinical trials are deemed as a gold standard method for analyzing and evaluating the safety and effectiveness of medical devices or pharmaceutical drugs.
•The most challenging part of conducting a randomized clinical trial are related to handling ethical and regulatory systems.
To Continue Reading : https://bit.ly/3eypDDm
Contact Us:
Website : https://bit.ly/3fQY0p0
Email Id: sales.cro@pepgra.com
Pharmaceutical legislation on notice to applicants and regulatory guidelines ...

1. Notice to Applicants
2. Volume 2
3. Volume 2A – Marketing Authorization
4. Volume 2B: Format and Presentation
5. Volume 2C: Regulatory Guideline
6. Concluison
To Continue Reading : https://bit.ly/3c4wu5p
Contact us;
website: https://bit.ly/2W1nV6r
Email: sales.cro@pepgra.com
Risk managements documents required for the market placement of a medical dev...

• The necessity of the risk management plan (RMP) has been studied before the launch of the medical device and medicinal product.
• Risk management documents/plan for medical device is done and verified through FDA QS regulations and ISO 14971.
• For medicinal products the risk management documents/plan is achieved by
• If more than one medicinal product is studied, article 14(2) of Regulation (EC) No 1394/2007 provides a layout for RMP for such advanced therapy medicinal products (ATMP)
To Continue reading : https://bit.ly/3e1harA
Contact us;
website: https://bit.ly/2W1nV6r
Email: sales.cro@pepgra.com
A few dedicated search engines for your medical writing

A medical literature search engine is a centralized browser-based platform which will come up with literature related to any of the medical subjects you choose. This is perhaps not a good idea to seek help of every medical resources discussed in this blog, yet using a few of the medical literature search engines will definitely serve the purpose of your constant source of authentic evidence.
To Continue Reading : https://bit.ly/35KkzYL
Medical literature monitoring and entering negative reaction reports

1. A novel process where European Medical Agency offers a new service.
2. The service is focused around medical literature monitoring.
3. This service is also a vital step to ensure that there is no duplication of negative reaction reports.
4. This service came into effect from 1st September, 2015.
To Continue Reading : https://bit.ly/39C4iVW
Pharmacovigilance Literature Search Services - https://bit.ly/2wM7IIH
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
Whatsapp: +91 9884350006
New medical device regulation: implications for medical device manufacturers

Pepgra offers regulatory consulting for Medical device and IVD companies. We help you in Market entry strategy, risk management, device classification and clinical evaluation reports. Let our team of regulatory experts work to ensure your compliance with all national regulations.
Learn More: https://www.pepgra.com/device-manufacturers/
Need Help:
Uk: +44- 7424810299
Email: sales@pepgra.com
Whatsapp: +91 9884350006
New Medical Device Regulation Implications For Medical Device Manufacturers

Pepgra offers regulatory consulting for Medical device and IVD companies. We help you in Market entry strategy, risk management, device classification and clinical evaluation reports. Let our team of regulatory experts work to ensure your compliance with all national regulations.
Learn more: https://www.pepgra.com/device-manufacturers/
Need Help:
Uk: +44- 7424810299
Email: sales@pepgra.com
Whatsapp: +91 9884350006
Relevant Medical Databases and Search Engines for Literature Screening

A medical literature search engine is a centralized browser-based platform which will come up with literature related to any of the medical subjects you choose. These search engines are programmed to be connected with the archives of published literature that are stored inside online subject-specific academic databases like the medical literature database.
Learn More: https://www.pepgra.com/relevant-medical-databases-and-search-engines-for-periodic-literature-screening/
Need Help:
Uk: +44- 7424810299
Email: sales@pepgra.com
Whatsapp: +91 9884350006
Pepgra Clinical Research Organisation

Pepgra Healthcare is a leading Contract Research Organization (CRO) assisting pharmaceutical, biotechnology, and medical
device manufacturers; we have offices in the US, UK, and India. Our CRO experts support all phases of clinical trials. Clinical
research, regulatory affairs, medical writing, Clinical Trial Protocols (CTP), biostatistical programming, trial patient monitoring,
therapeutics, Post-Marketing Surveillance (PMS) and more. Pepgra is 9001:2015 certified organization.
In addition, Pepgra’s publication division focuses on academic and scientific writing projects. Editing, publication, research,
physician writing, and scientific communication—these are our focus areas.
More from PEPGRA Healthcare (20)
Clinical Research Organization Services | Contract Research Company - Pepgra

Clinical Research Organization Services | Contract Research Company - Pepgra
Different Stages of Medical Device Development and Drug Development: PepgraDi...

Different Stages of Medical Device Development and Drug Development: PepgraDi...
Top 5 tips for managing risks in your clinical studies - Pepgra

Top 5 tips for managing risks in your clinical studies - Pepgra
Role of Biostatistician and Biostatistical Programming in Epidemiological Stu...

Role of Biostatistician and Biostatistical Programming in Epidemiological Stu...
EMA Guidelines for Clinical Trial Management - Pepgra Healthcare

EMA Guidelines for Clinical Trial Management - Pepgra Healthcare
Side effects of drugs products, medical devices & drugs healthcare data...

Side effects of drugs products, medical devices & drugs healthcare data...
Guidance for Biomarkers into Early Phase Clinical Research Purposes | Healthc...

Guidance for Biomarkers into Early Phase Clinical Research Purposes | Healthc...
Recent trends in genomic biomarkers pepgra healthcare

Recent trends in genomic biomarkers pepgra healthcare
Recent Trends in Genomic Biomarkers - Pepgra Healthcare

Recent Trends in Genomic Biomarkers - Pepgra Healthcare
Guidelines on Virtuous Pharmacovigilance Practices | Pepgra Healthcare

Guidelines on Virtuous Pharmacovigilance Practices | Pepgra Healthcare
Challenges and-opportunities-in-software-driven-medical-sciences

Challenges and-opportunities-in-software-driven-medical-sciences
Clinical trail-designchallenges-in-the-study-design-conduct-and-analysis-of-r...

Clinical trail-designchallenges-in-the-study-design-conduct-and-analysis-of-r...
Pharmaceutical legislation on notice to applicants and regulatory guidelines ...

Pharmaceutical legislation on notice to applicants and regulatory guidelines ...
Risk managements documents required for the market placement of a medical dev...

Risk managements documents required for the market placement of a medical dev...
A few dedicated search engines for your medical writing

A few dedicated search engines for your medical writing
Medical literature monitoring and entering negative reaction reports

Medical literature monitoring and entering negative reaction reports
New medical device regulation: implications for medical device manufacturers

New medical device regulation: implications for medical device manufacturers
New Medical Device Regulation Implications For Medical Device Manufacturers

New Medical Device Regulation Implications For Medical Device Manufacturers
Relevant Medical Databases and Search Engines for Literature Screening

Relevant Medical Databases and Search Engines for Literature Screening
Recently uploaded
The positive impact of SGRT – The Berkshire Cancer Centre experience

SGRT Europe 2023
Victoria Hammond-Turner
Technical and Development Lead Therapeutic Radiographer
Royal Berkshire NHS Foundation Trust, UK
Veterinary Diagnostics Market PPT 2024: Size, Growth, Demand and Forecast til...

The global veterinary diagnostics market size reached US$ 6.6 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 12.6 Billion by 2032, exhibiting a growth rate (CAGR) of 7.3% during 2024-2032.
More Info:- https://www.imarcgroup.com/veterinary-diagnostics-market
Mastoid cavity problem and obilteration presentation by Dr Salison Salim Pani...

Mastoid surgery cavity problems presentation by Dr Salison Salim Panicker, ENT surgeon, RelentCare, Kolazhy, Thrissur.
Dimensions of Healthcare Quality

The dimensions of healthcare quality refer to various attributes or aspects that define the standard of healthcare services. These dimensions are used to evaluate, measure, and improve the quality of care provided to patients. A comprehensive understanding of these dimensions ensures that healthcare systems can address various aspects of patient care effectively and holistically. Dimensions of Healthcare Quality and Performance of care include the following; Appropriateness, Availability, Competence, Continuity, Effectiveness, Efficiency, Efficacy, Prevention, Respect and Care, Safety as well as Timeliness.
Cold Sores: Causes, Treatments, and Prevention Strategies | The Lifesciences ...

Cold Sores: Causes, Treatments, and Prevention Strategies | The Lifesciences ...The Lifesciences Magazine
Cold Sores, medically known as herpes labialis, are caused by the herpes simplex virus (HSV). HSV-1 is primarily responsible for cold sores, although HSV-2 can also contribute in some cases.CANSA support - Caring for Cancer Patients' Caregivers

International Cancer Survivors Day is celebrated during June, placing the spotlight not only on cancer survivors, but also their caregivers.
CANSA has compiled a list of tips and guidelines of support:
https://cansa.org.za/who-cares-for-cancer-patients-caregivers/
CHAPTER 1 SEMESTER V PREVENTIVE-PEDIATRICS.pdf

This content provides an overview of preventive pediatrics. It defines preventive pediatrics as preventing disease and promoting children's physical, mental, and social well-being to achieve positive health. It discusses antenatal, postnatal, and social preventive pediatrics. It also covers various child health programs like immunization, breastfeeding, ICDS, and the roles of organizations like WHO, UNICEF, and nurses in preventive pediatrics.
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

This webinar helps clinicians understand the unique healthcare needs of the LGBTQ+ community, primarily in relation to end-of-life care. Topics include social and cultural background and challenges, healthcare disparities, advanced care planning, and strategies for reaching the community and improving quality of care.
Feeding plate for a newborn with Cleft Palate.pptx

A feeding plate is a prosthetic device used for newborns with a cleft palate to assist in feeding and improve nutrition intake. From a prosthodontic perspective, this plate acts as a barrier between the oral and nasal cavities, facilitating effective sucking and swallowing by providing a more normal anatomical structure. It helps to prevent milk from entering the nasal passage, thereby reducing the risk of aspiration and enhancing the infant's ability to feed efficiently. The feeding plate also aids in the development of the oral muscles and can contribute to better growth and weight gain. Its custom fabrication and proper fitting by a prosthodontist are crucial for ensuring comfort and functionality, as well as for minimizing potential complications. Early intervention with a feeding plate can significantly improve the quality of life for both the infant and the parents.
Artificial Intelligence to Optimize Cardiovascular Therapy

Presentation at the annual convention of the Philippine Heart Association, 31 May 2024. EDSA Shangrila Hotel, Manila.
INFECTION OF THE BRAIN -ENCEPHALITIS ( PPT)

Neurological system includes brain and spinal cord. It plays an important role in functioning of our body. Encephalitis is the inflammation of the brain. Causes include viral infections, infections from insect bites or an autoimmune reaction that affects the brain. It can be life-threatening or cause long-term complications. Treatment varies, but most people require hospitalization so they can receive intensive treatment, including life support.
Navigating Challenges: Mental Health, Legislation, and the Prison System in B...

This conference will delve into the intricate intersections between mental health, legal frameworks, and the prison system in Bolivia. It aims to provide a comprehensive overview of the current challenges faced by mental health professionals working within the legislative and correctional landscapes. Topics of discussion will include the prevalence and impact of mental health issues among the incarcerated population, the effectiveness of existing mental health policies and legislation, and potential reforms to enhance the mental health support system within prisons.
Luxurious Spa In Ajman Chandrima Massage Center

Chandrima Spa Ajman is one of the leading Massage Center in Ajman, which is open 24 hours exclusively for men. Being one of the most affordable Spa in Ajman, we offer Body to Body massage, Kerala Massage, Malayali Massage, Indian Massage, Pakistani Massage Russian massage, Thai massage, Swedish massage, Hot Stone Massage, Deep Tissue Massage, and many more. Indulge in the ultimate massage experience and book your appointment today. We are confident that you will leave our Massage spa feeling refreshed, rejuvenated, and ready to take on the world.
Visit : https://massagespaajman.com/
Call : 052 987 1315
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx ensures effective, cost-efficient medication management for chronic diseases, enhancing patient health and productivity.
一比一原版纽约大学毕业证(NYU毕业证)成绩单留信认证

原版定制【微信:41543339】【纽约大学毕业证(NYU毕业证)】【微信:41543339】成绩单、外壳、offer、留信学历认证(永久存档真实可查)采用学校原版纸张、特殊工艺完全按照原版一比一制作(包括:隐形水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠,文字图案浮雕,激光镭射,紫外荧光,温感,复印防伪)行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备,十五年致力于帮助留学生解决难题,业务范围有加拿大、英国、澳洲、韩国、美国、新加坡,新西兰等学历材料,包您满意。
【我们承诺采用的是学校原版纸张(纸质、底色、纹路),我们拥有全套进口原装设备,特殊工艺都是采用不同机器制作,仿真度基本可以达到98%以上,所有工艺效果都可提前给客户展示,不满意可以根据客户要求进行调整,直到满意为止!】
【业务选择办理准则】
一、工作未确定,回国需先给父母、亲戚朋友看下文凭的情况,办理一份就读学校的毕业证【微信41543339】文凭即可
二、回国进私企、外企、自己做生意的情况,这些单位是不查询毕业证真伪的,而且国内没有渠道去查询国外文凭的真假,也不需要提供真实教育部认证。鉴于此,办理一份毕业证【微信41543339】即可
三、进国企,银行,事业单位,考公务员等等,这些单位是必需要提供真实教育部认证的,办理教育部认证所需资料众多且烦琐,所有材料您都必须提供原件,我们凭借丰富的经验,快捷的绿色通道帮您快速整合材料,让您少走弯路。
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
留信网服务项目:
1、留学生专业人才库服务(留信分析)
2、国(境)学习人员提供就业推荐信服务
3、留学人员区块链存储服务
→ 【关于价格问题(保证一手价格)】
我们所定的价格是非常合理的,而且我们现在做得单子大多数都是代理和回头客户介绍的所以一般现在有新的单子 我给客户的都是第一手的代理价格,因为我想坦诚对待大家 不想跟大家在价格方面浪费时间
对于老客户或者被老客户介绍过来的朋友,我们都会适当给一些优惠。
选择实体注册公司办理,更放心,更安全!我们的承诺:客户在留信官方认证查询网站查询到认证通过结果后付款,不成功不收费!
RECENT ADVANCES IN BREAST CANCER RADIOTHERAPY

ULTRA HYPOFRACTIONATION
Accelerated Partial Breast Irradiation: :Brachytherapy
UK FAST FORWARD
Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...The Lifesciences Magazine
Deep Leg Vein Thrombosis occurs when a blood clot forms in one or more of the deep veins in the legs. These clots can impede blood flow, leading to severe complications.Top massage center in ajman chandrima Spa

We are one of the top Massage Spa Ajman Our highly skilled, experienced, and certified massage therapists from different corners of the world are committed to serving you with a soothing and relaxing experience. Luxuriate yourself at our spas in Sharjah and Ajman, which are indeed enriched with an ambiance of relaxation and tranquility. We could confidently claim that we are one of the most affordable Spa Ajman and Sharjah as well, where you can book the massage session of your choice for just 99 AED at any time as we are open 24 hours a day, 7 days a week.
Visit : https://massagespaajman.com/
Call : 052 987 1315
ICH Guidelines for Pharmacovigilance.pdf

The "ICH Guidelines for Pharmacovigilance" PDF provides a comprehensive overview of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines related to pharmacovigilance. These guidelines aim to ensure that drugs are safe and effective for patients by monitoring and assessing adverse effects, ensuring proper reporting systems, and improving risk management practices. The document is essential for professionals in the pharmaceutical industry, regulatory authorities, and healthcare providers, offering detailed procedures and standards for pharmacovigilance activities to enhance drug safety and protect public health.
Recently uploaded (20)
The positive impact of SGRT – The Berkshire Cancer Centre experience

The positive impact of SGRT – The Berkshire Cancer Centre experience
Veterinary Diagnostics Market PPT 2024: Size, Growth, Demand and Forecast til...

Veterinary Diagnostics Market PPT 2024: Size, Growth, Demand and Forecast til...
Mastoid cavity problem and obilteration presentation by Dr Salison Salim Pani...

Mastoid cavity problem and obilteration presentation by Dr Salison Salim Pani...
Cold Sores: Causes, Treatments, and Prevention Strategies | The Lifesciences ...

Cold Sores: Causes, Treatments, and Prevention Strategies | The Lifesciences ...
CANSA support - Caring for Cancer Patients' Caregivers

CANSA support - Caring for Cancer Patients' Caregivers
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care
Feeding plate for a newborn with Cleft Palate.pptx

Feeding plate for a newborn with Cleft Palate.pptx
Artificial Intelligence to Optimize Cardiovascular Therapy

Artificial Intelligence to Optimize Cardiovascular Therapy
Navigating Challenges: Mental Health, Legislation, and the Prison System in B...

Navigating Challenges: Mental Health, Legislation, and the Prison System in B...
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx's Function in the Management of Chronic Illnesses
Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...
Clinical Trial Design and Artificial Intelligence | Pepgra.com
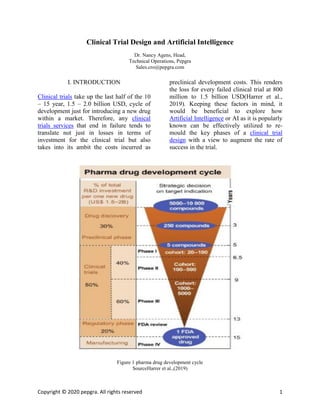
- 1. Copyright © 2020 pepgra. All rights reserved 1 Clinical Trial Design and Artificial Intelligence Dr. Nancy Agens, Head, Technical Operations, Pepgra Sales.cro@pepgra.com I. INTRODUCTION Clinical trials take up the last half of the 10 – 15 year, 1.5 – 2.0 billion USD, cycle of development just for introducing a new drug within a market. Therefore, any clinical trials services that end in failure tends to translate not just in losses in terms of investment for the clinical trial but also takes into its ambit the costs incurred as preclinical development costs. This renders the loss for every failed clinical trial at 800 million to 1.5 billion USD(Harrer et al., 2019). Keeping these factors in mind, it would be beneficial to explore how Artificial Intelligence or AI as it is popularly known can be effectively utilized to re- mould the key phases of a clinical trial design with a view to augment the rate of success in the trial. Figure 1 pharma drug development cycle SourceHarrer et al.,(2019)
- 2. Copyright © 2020 pepgra. All rights reserved 2 II. FACTORS CAUSING FAILURES IN CLINICAL TRIALS AND HOW AI CAN HELP The main two factors that lead to failure within clinical trials services pertains to the selection of patient cohorts and the mechanisms used for recruiting cohorts. The said two factors are not enough to bring in the most appropriate patients to the trial in a timely manner, along with the absence of a technical infrastructure to match the intricacies of executing a clinical trial(Fogel, 2018). This is particularly true in phases that come later particularly, where there is a lack of an effective and reliable protocol for adherence, clinical endpoint detection systems and patient monitoring, In such scenarios, AI can prove to be extremely beneficial in circumventing such shortfalls of the present clinical trial design. Specifically, deep learning and machine learning would be in a position to automatically seek out meaningful patterns from huge datasets such as speech, text or images. Natural language processing (NLP) as well as Human Machine Interfaces (HMIs) enables a seamless transfer of information amongst humans and computers(Nadkarni et al., 2011). III. AI AND ITS EVOLUTION AI in the domain of medicine has a history which goes back to the early 1970s when proficient systems such as MYCIN had been initially introduced for providing support to diagnostic decisions(Shortliffe, 1984). Nonetheless, early systems of medical AI depended largely on experts from the medical domain for training computers by encrypting clinical knowledge as logic based rules for particular situations or scenarios. Systems of such kind were wrought with the limitation wherein it was intensive to labour and required a lot of time for the construction process. Also, once it was developed there were also considerable challenges with regards to any future updates as such systems were largely rigid(McCauley & Ala, 1992). Highly advanced systems of machine learning which had the ability to train itself to imbibe such rules by recognizing and weighing in pertinent aspects from data like medical images, unstructured texts and electronic health records made an entrance only in the 90s and 2000s. However, the medical domain was slow in terms of adapting such systems mainly owing to the absence of data that was extensively available. This was further aggravated by the fact that such early techniques warranted intense feature engineering efforts that involved severe commitments from experts within the medical domain(Niu et al., 2011). Over the years, the situation improved tremendously especially in recent times, mainly due to two key factors. First and foremost, the domain of AI as such experienced transformational developments, especially in deep learning and machine learning techniques which were facilitated through hardware enhancements and extensive datasets for training(Wang et al., 2019). Secondly, data in the medical domain became largely available and easily accessible in digital formats. Credit goes to innovative developments in technology and also to initiatives on public policy such as the Electronic Records Meaningful Use Programs within the United States of America. IV. AI IN CLINICAL TRIALS The figure above envisions the key techniques through which AI can be introduced within the tenets of clinical design. There are three key themes within the design which comprises of; cohort composition, patient recruitment and patient
- 3. Copyright © 2020 pepgra. All rights reserved 3 monitoring. These are founded on the features of the patient in terms of eligibility, suitability, empowerment, enrollment and motivation. This is also inclusive of aspects pertaining to trial such as; adherence control, endpoint detection and retention of patients. Figure 2 AI for clinical trial design SourceHarrer et al.,(2019) An array of methodologies for design has been utilized to execute functionalities of the target. Functionalities such as these are facilitated through distinct combinations from the three key AI technologies – deep / machine learning, human machine interfaces and reasoning wherein every single technology evaluates a particular set of patient and functionality specific sources of data. The pertinent enhancement which is introduced through such type of an execution on the outcome of the study is signified by the length of the horizontal lines within the color bar code beneath the key outcome features. Every study design application that is based on AI directly hinges on the quantum and quality of data it
- 4. Copyright © 2020 pepgra. All rights reserved 4 can tap into, and thus is confronted with the similar basic challenges. REFERENCES [1] Fogel, D.B. (2018). Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemporary Clinical Trials Communications. [Online]. 11. pp. 156–164. Available from: https://linkinghub.elsevier.com/retrieve/pii/S245186 5418300693. [2] Harrer, S., Shah, P., Antony, B. & Hu, J. (2019). Artificial Intelligence for Clinical Trial Design. Trends in Pharmacological Sciences. [Online]. 40 (8). pp. 577–591. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016561 4719301300. [3] McCauley, N. & Ala, M. (1992). The use of expert systems in the healthcare industry. Information & Management. [Online]. 22 (4). pp. 227–235. Available from: https://linkinghub.elsevier.com/retrieve/pii/0378720 69290025B. [4] Nadkarni, P.M., Ohno-Machado, L. & Chapman, W.W. (2011). Natural language processing: an introduction. Journal of the American Medical Informatics Association. [Online]. 18 (5). pp. 544– 551. Available from: https://academic.oup.com/jamia/article- lookup/doi/10.1136/amiajnl-2011-000464. [5] Niu, F., Recht, B., Re, C. & Wright, S.J. (2011). HOGWILD!: A Lock-Free Approach to Parallelizing Stochastic Gradient Descent. [Online]. Available from: http://arxiv.org/abs/1106.5730. [6] Shortliffe, W.J.C. and E.H. (1984). Readings in Medical Artificial Intelligence: The First Decade. [Online]. Available from: http://people.dbmi.columbia.edu/~ehs7001/Clancey- Shortliffe-1984/Readings Book.htm. [7] Wang, F., Casalino, L.P. & Khullar, D. (2019). Deep Learning in Medicine—Promise, Progress, and Challenges. JAMA Internal Medicine. [Online]. 179 (3). pp. 293. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10 .1001/jamainternmed.2018.7117.
