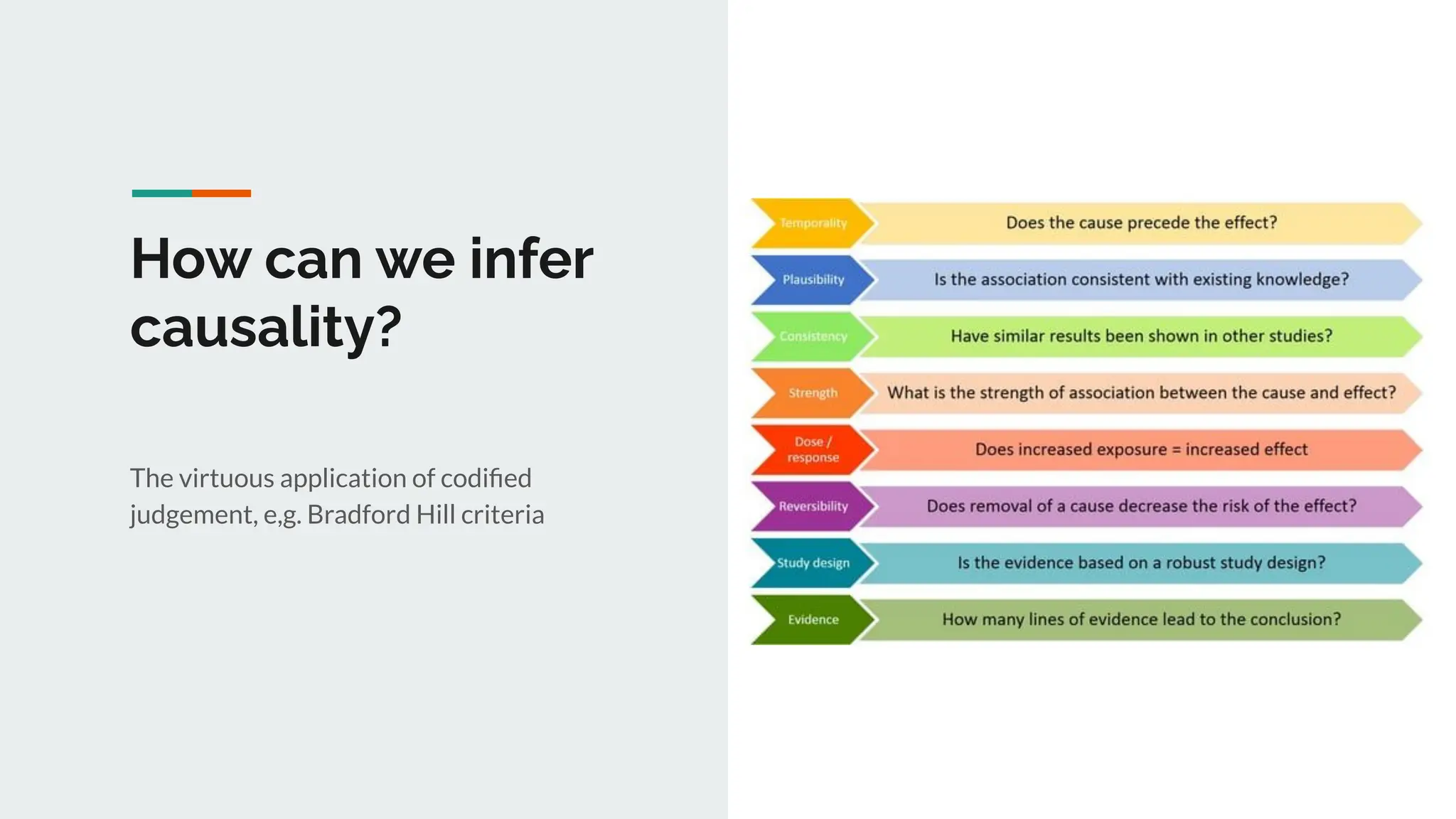
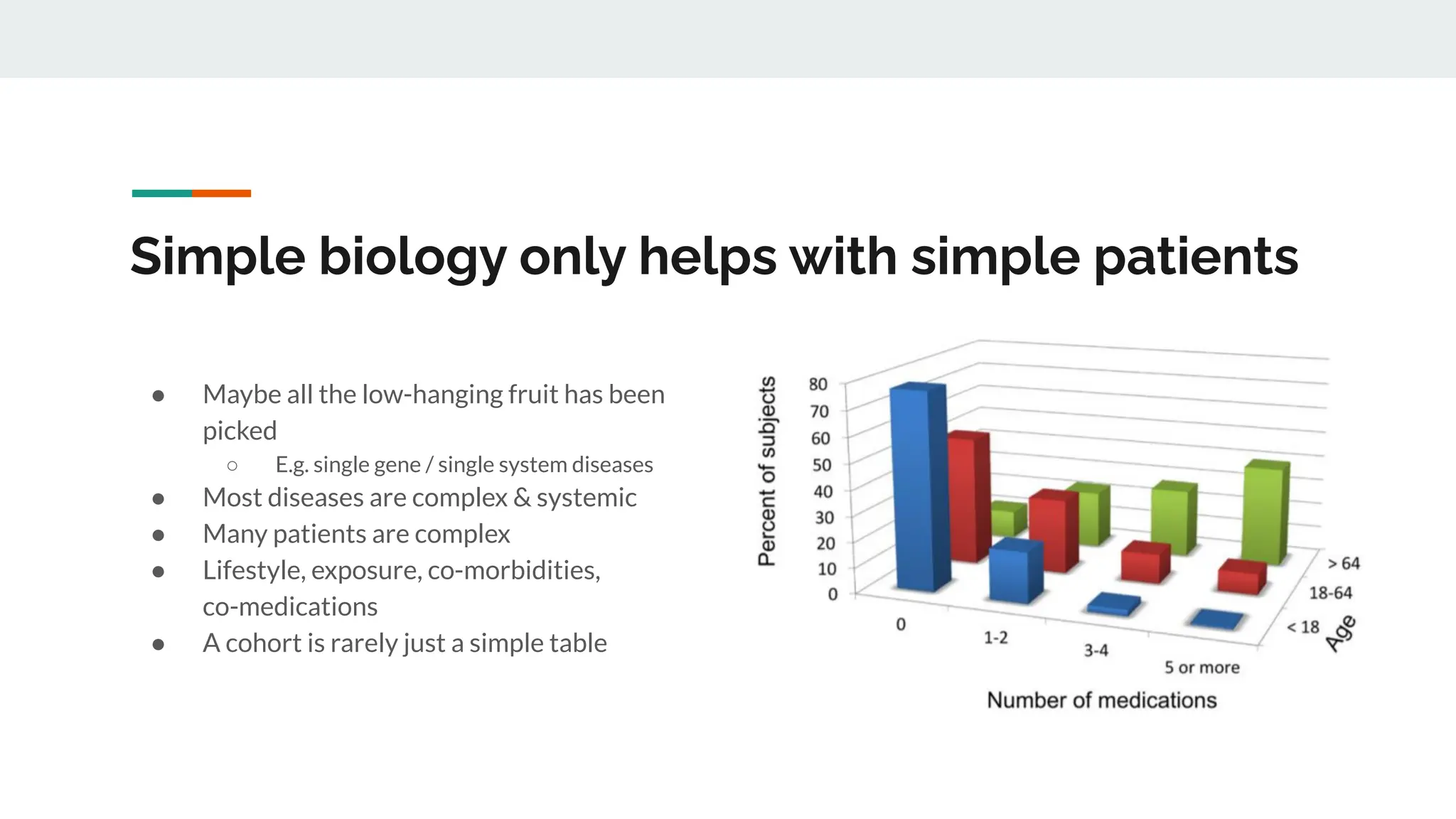
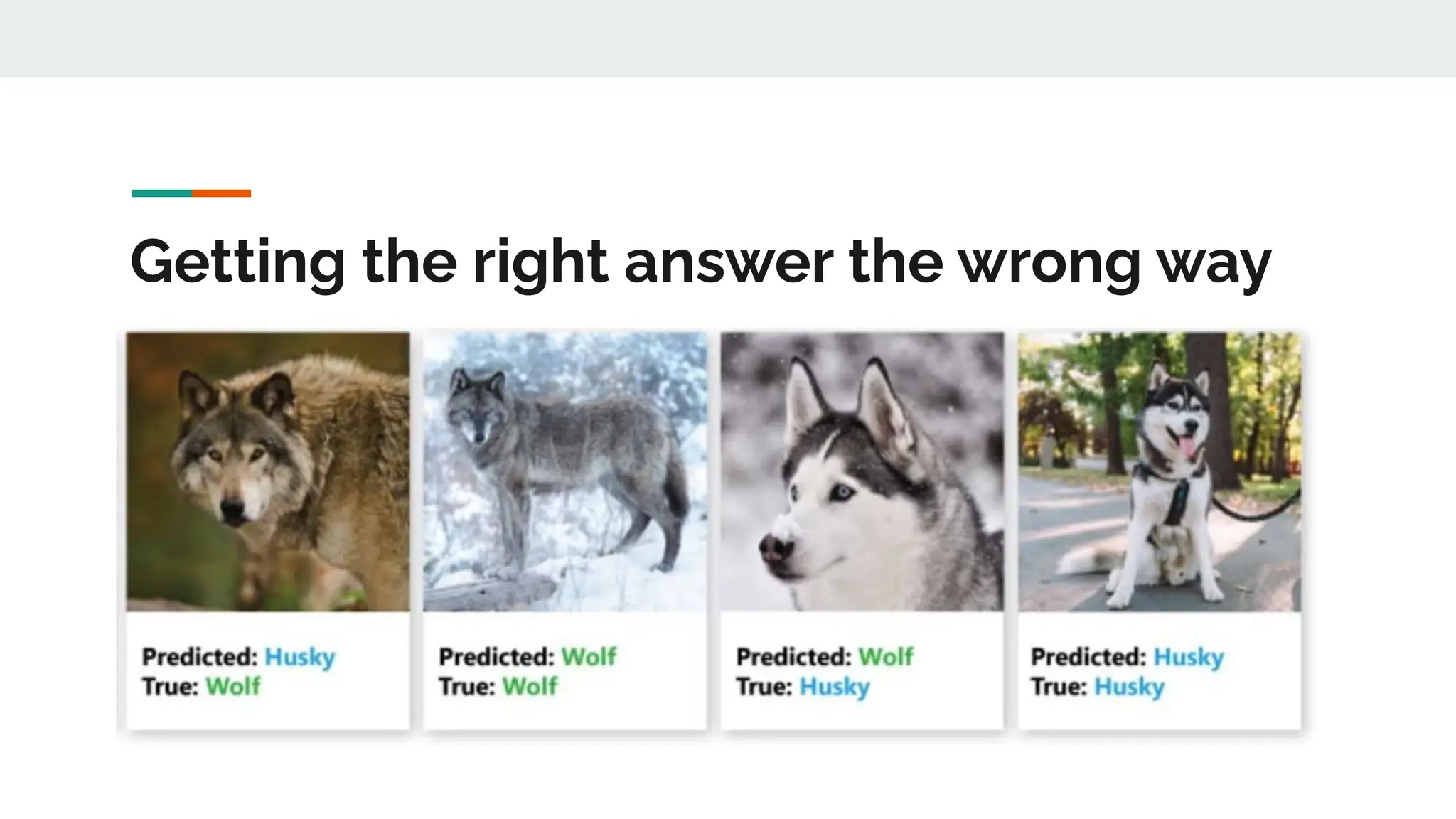
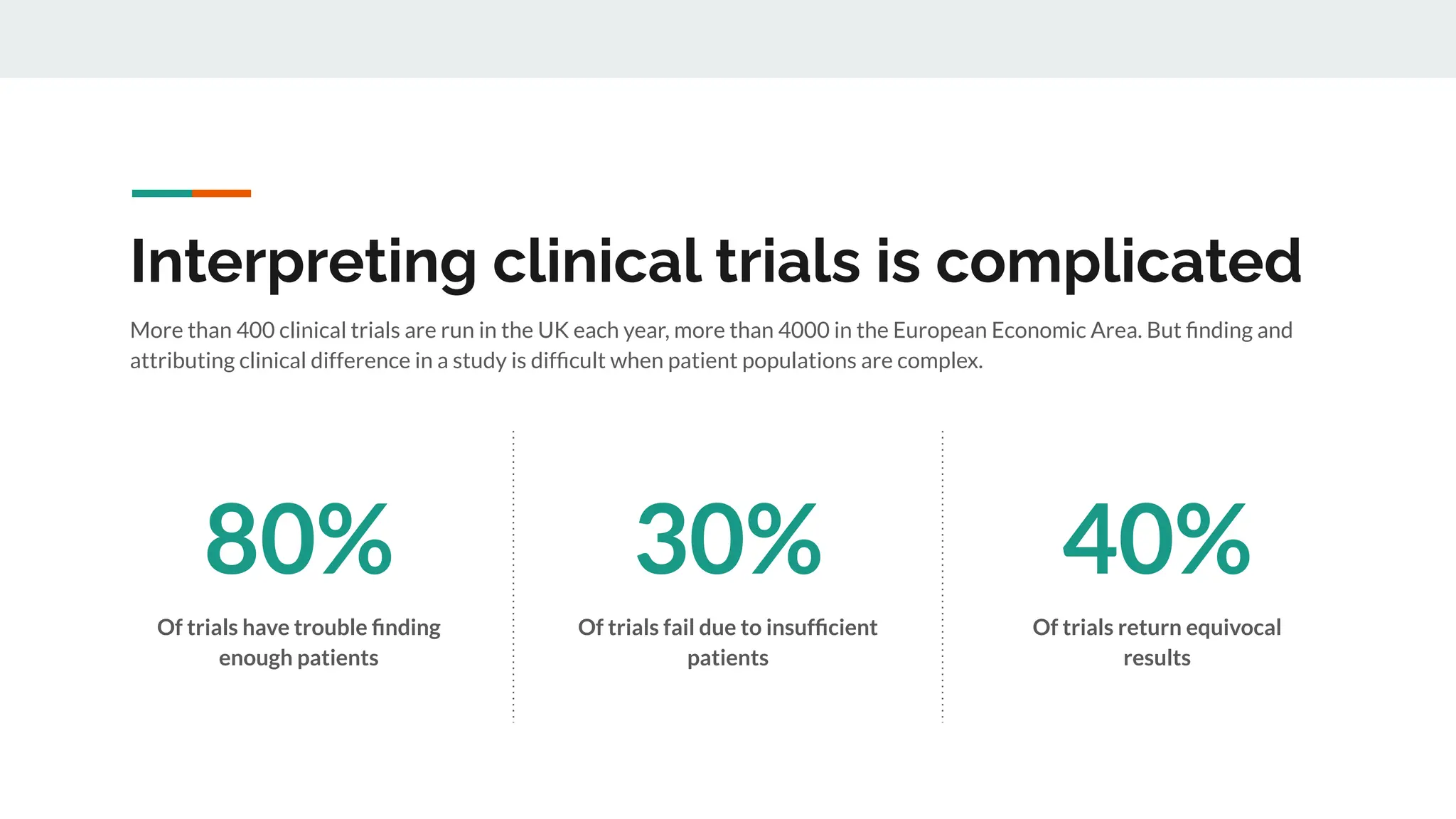
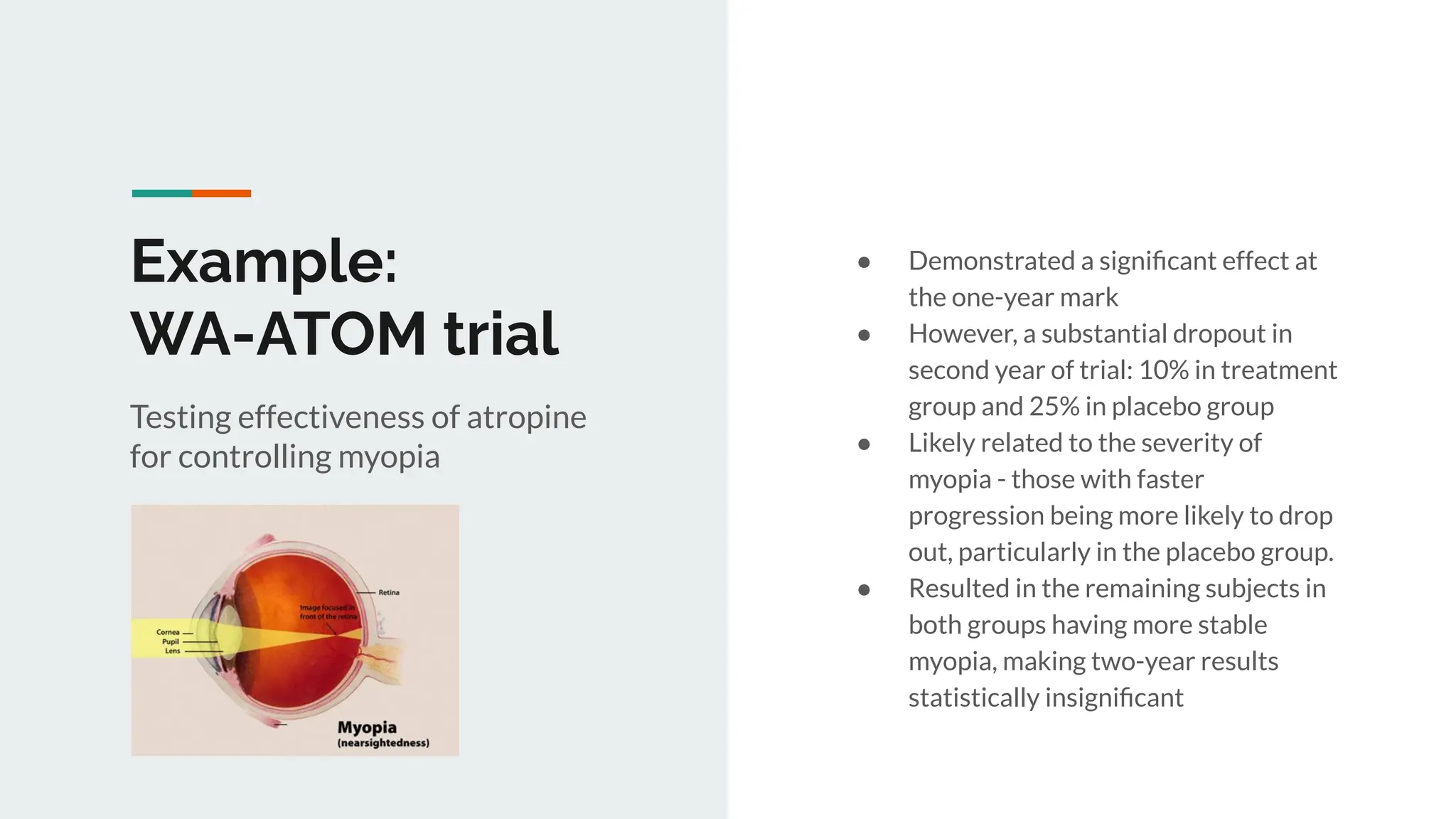
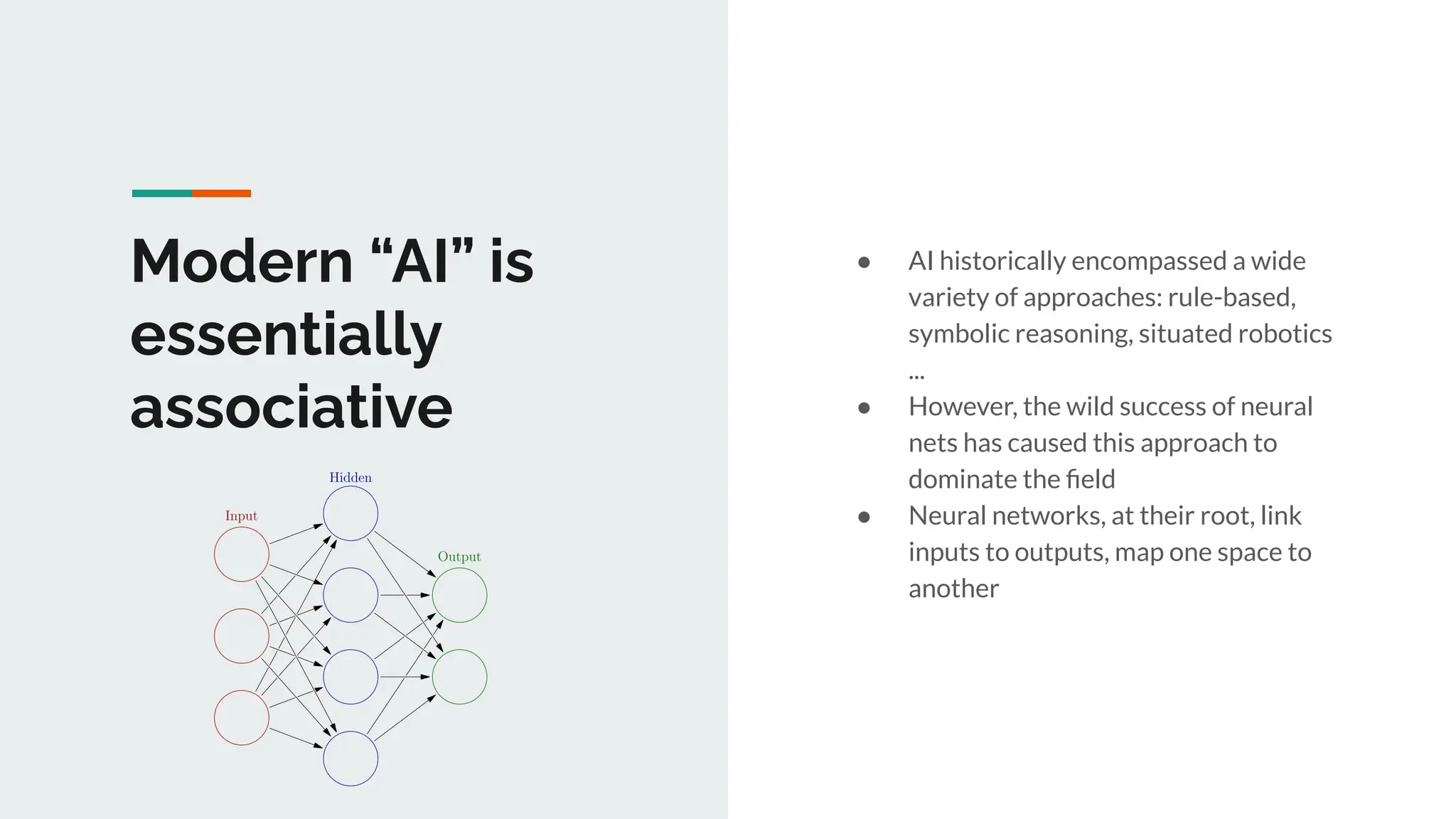
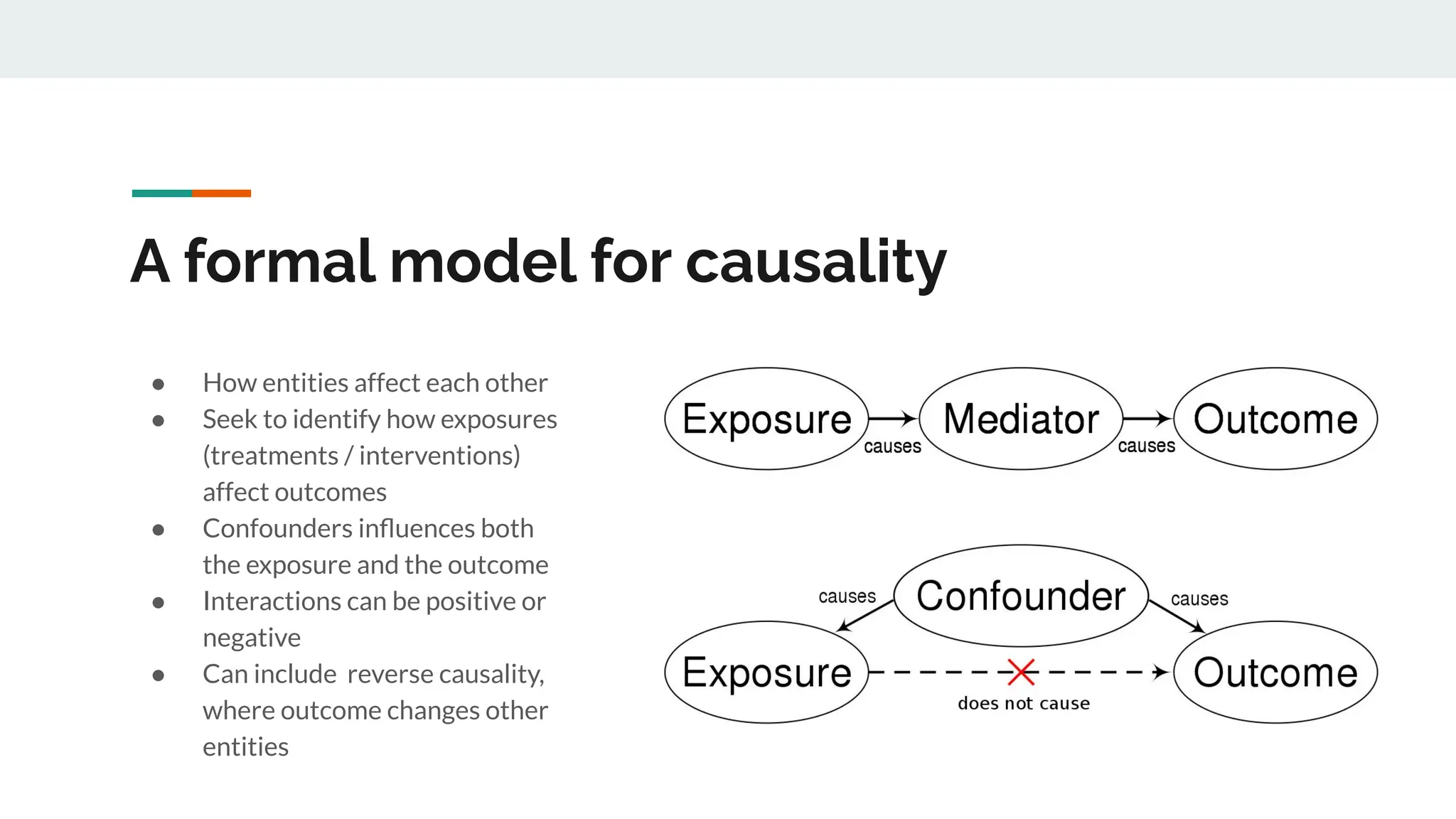
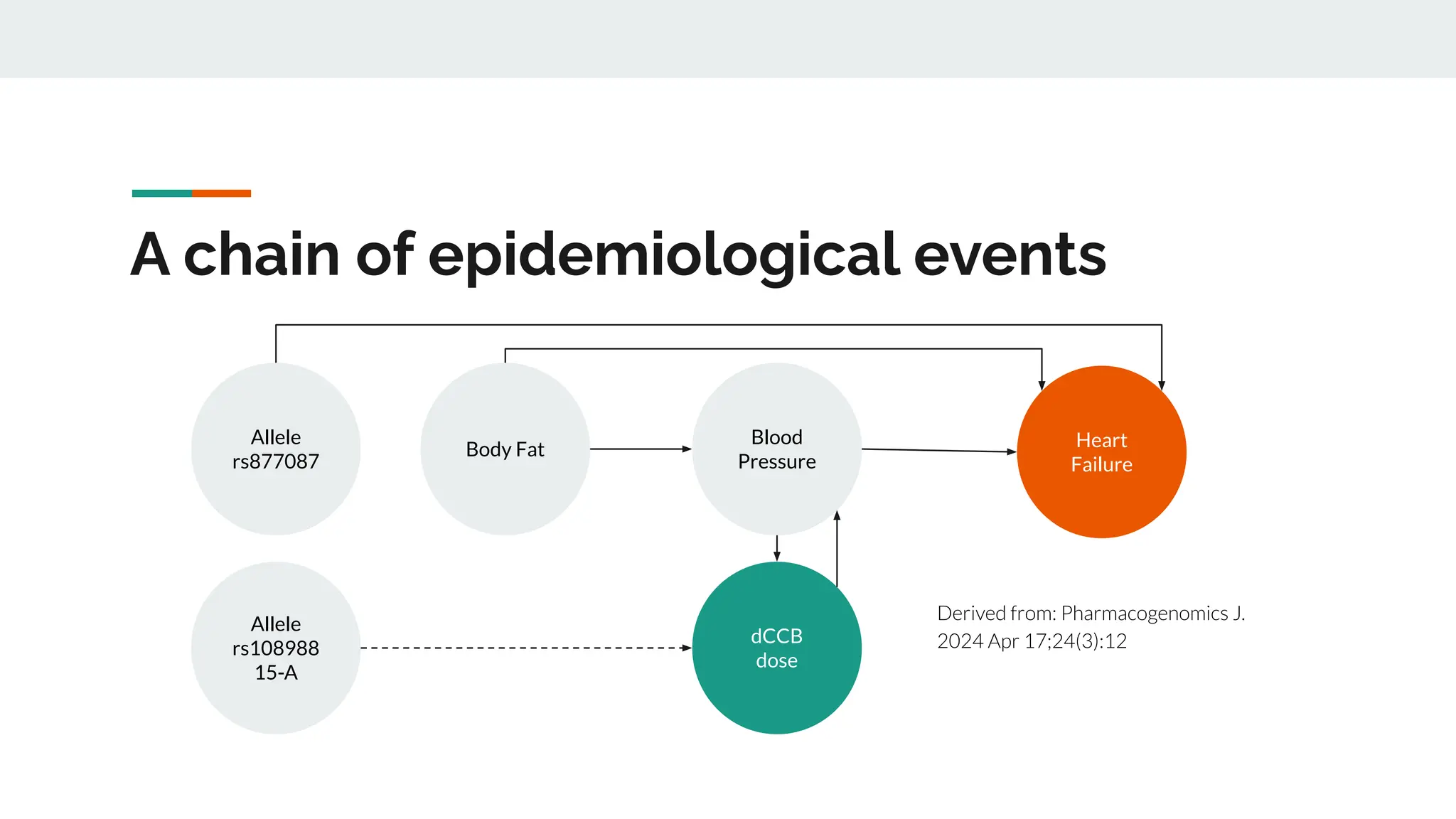
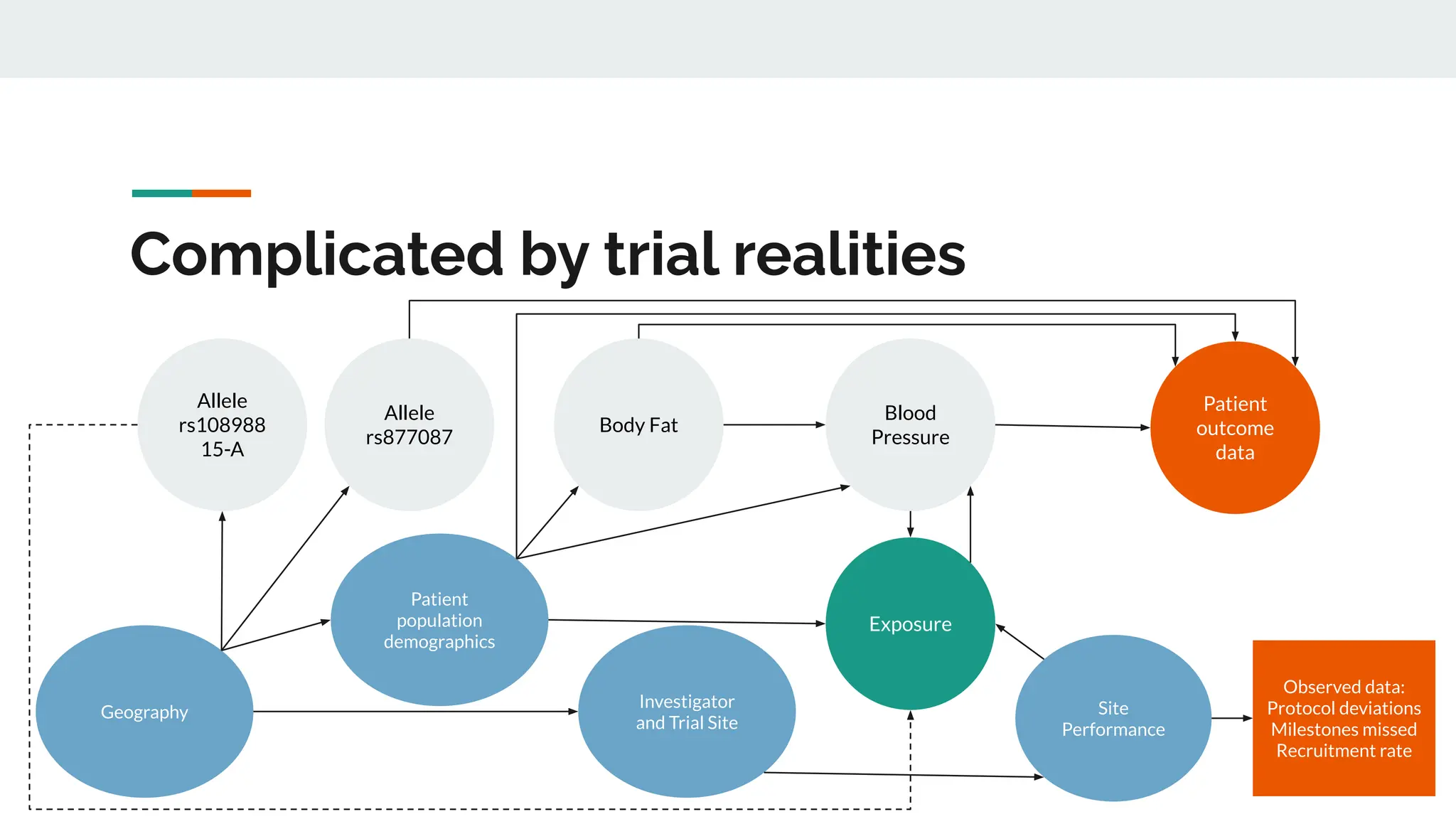
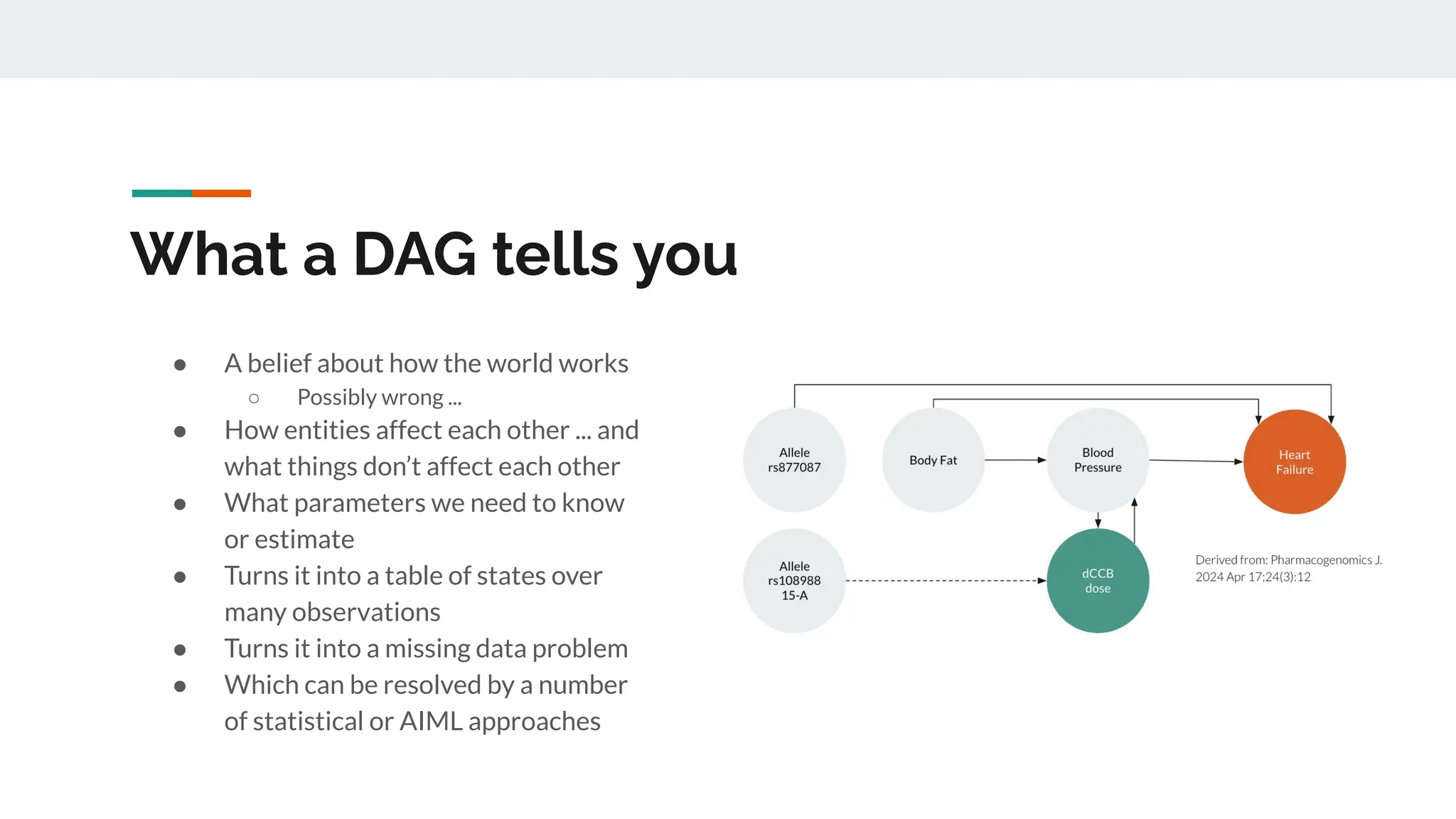
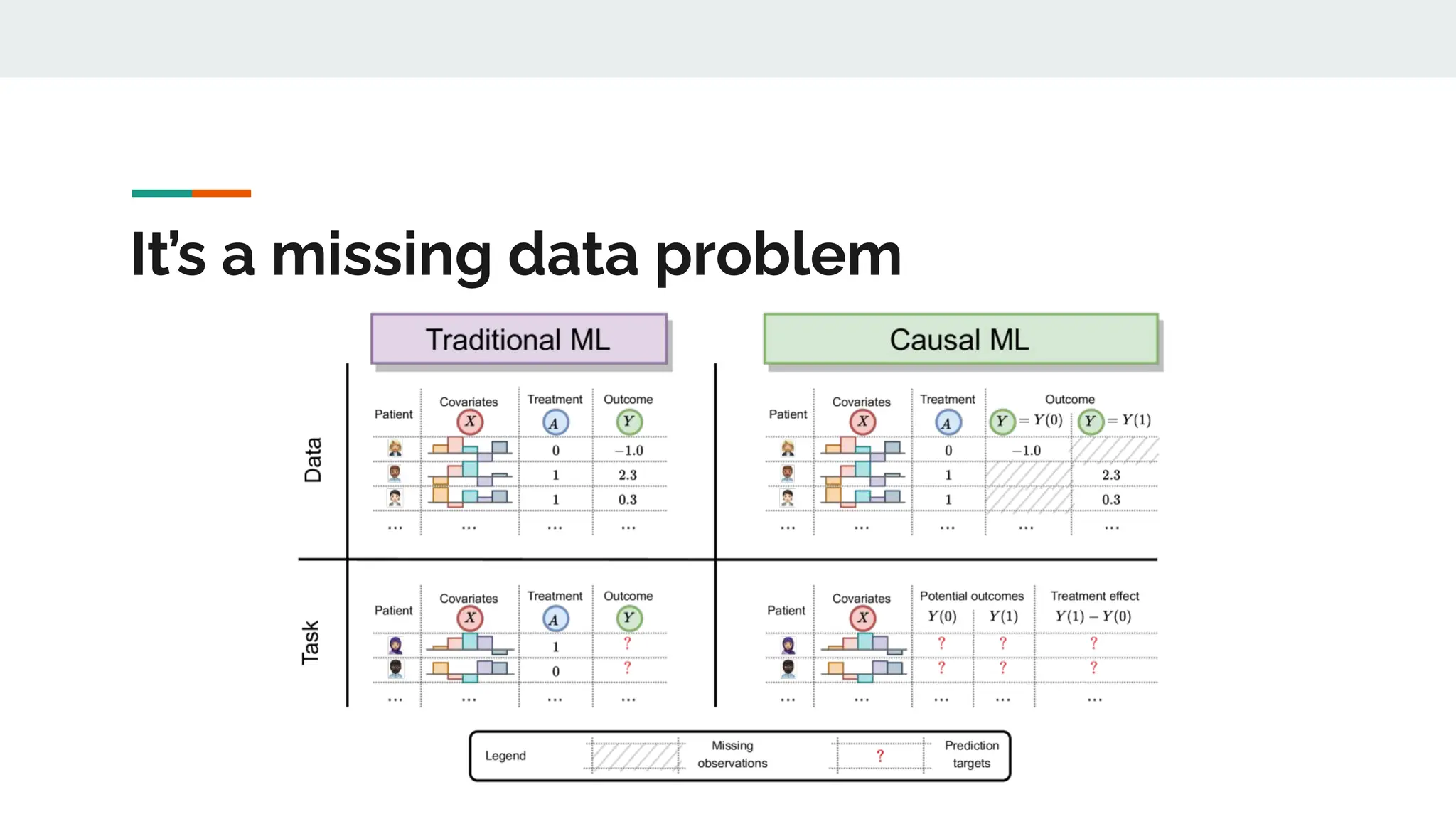
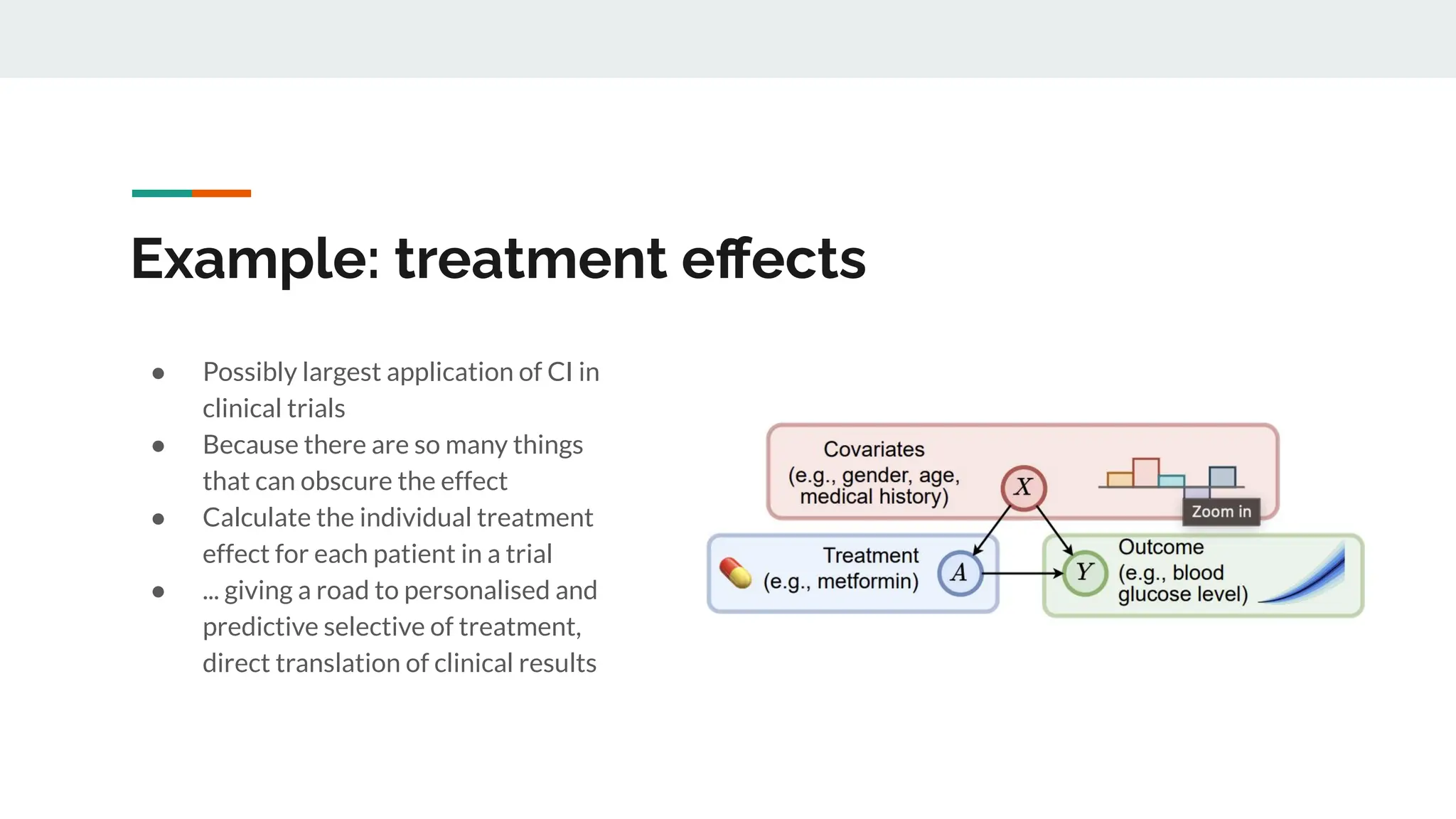
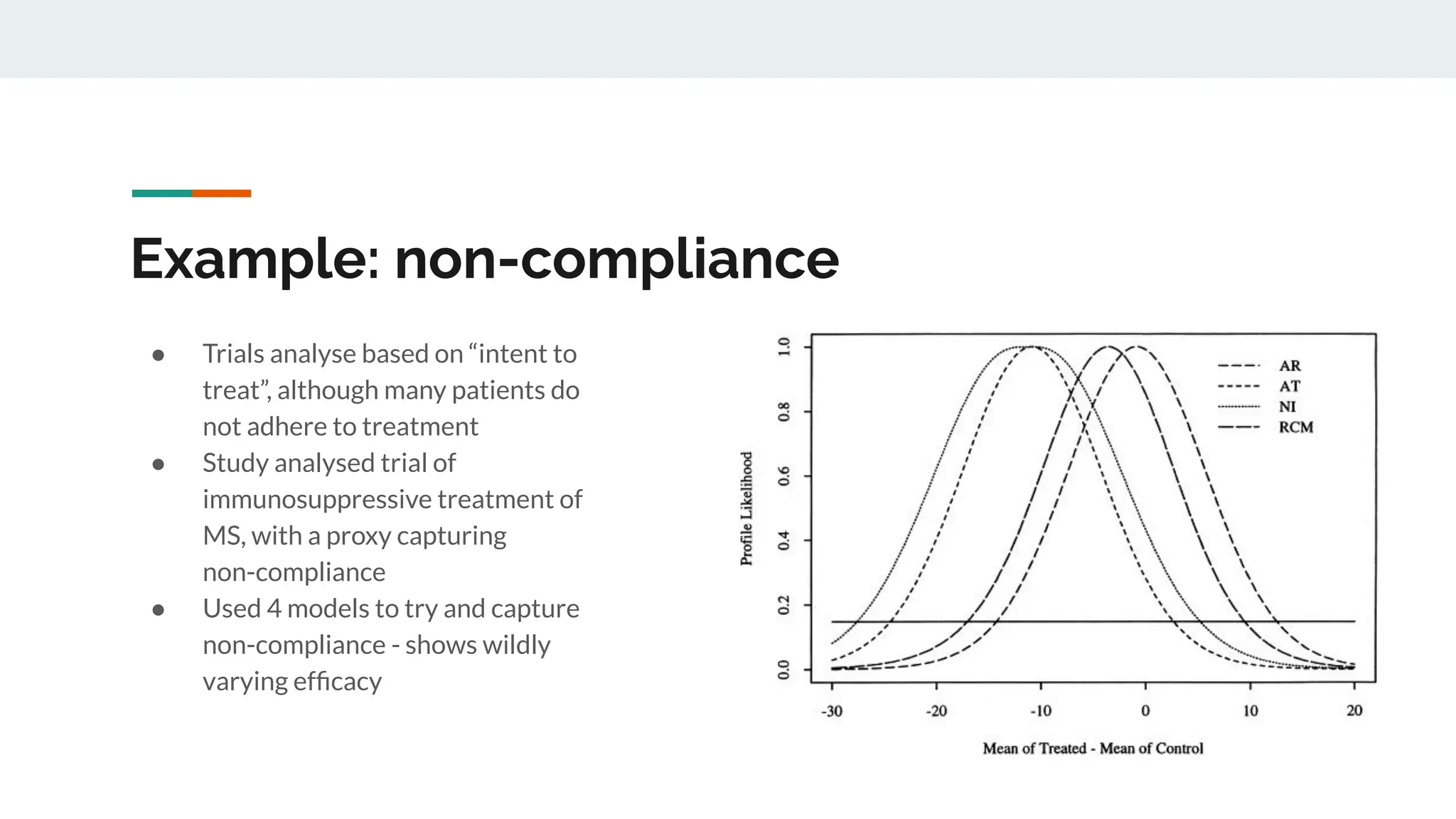
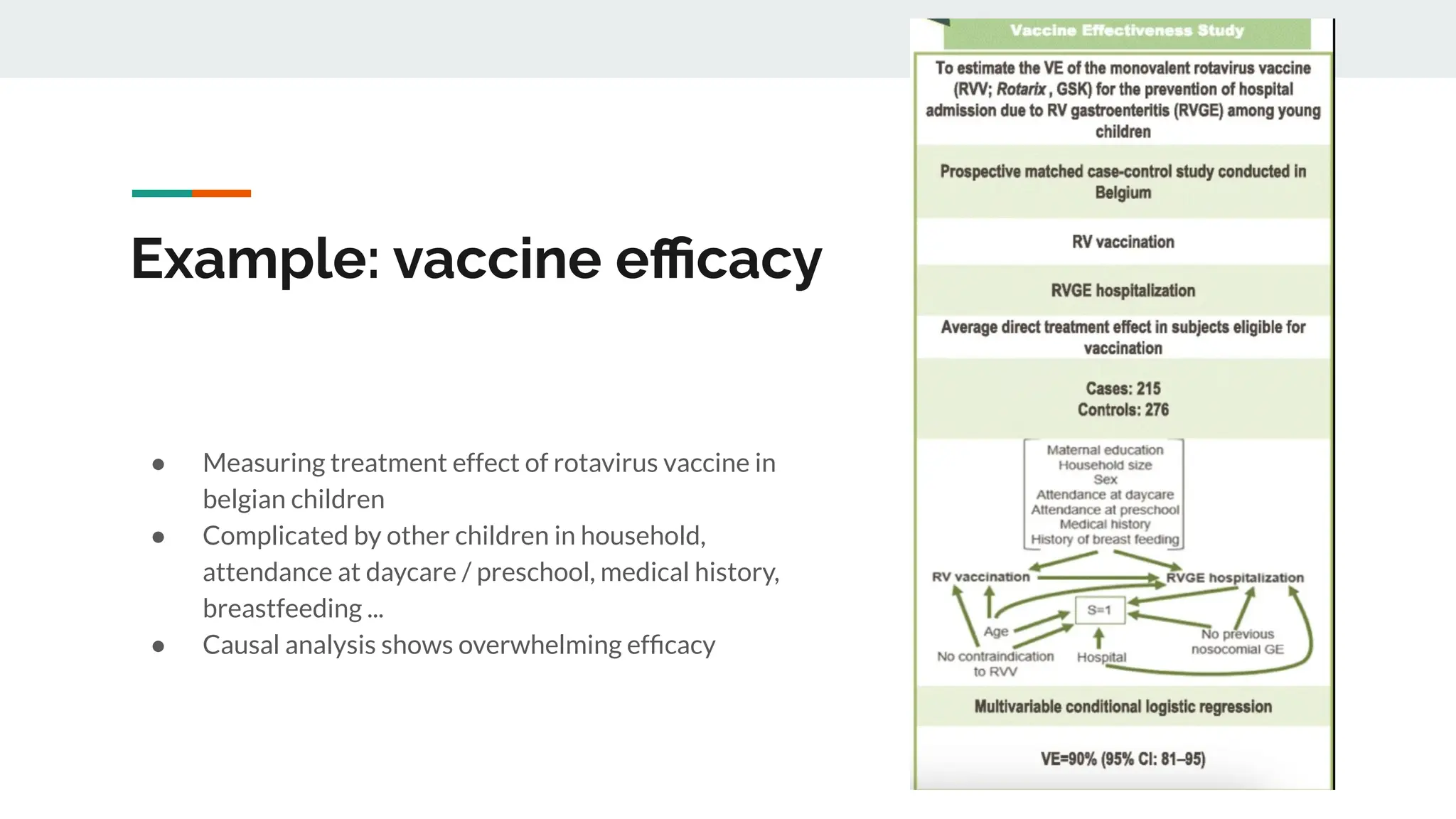
No therapy or medical device gets into use without going through a clinical trial. However trials are often poor at helping us to learn, and will return conflicted or ambiguous information. These problems are writ even larger with observational studies and Real World Data. Here, I explore whether causal analysis is the answer, helping us to get a less associative and more real picture of the world