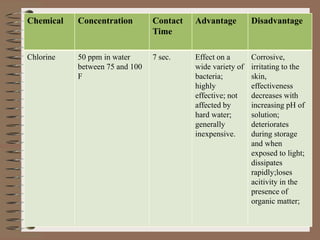
The document discusses cleaning and sanitizing in a restaurant kitchen. It defines cleaning as removing soil while sanitizing kills microorganisms. There are four categories of cleaning agents - detergents, solvents, acids, and abrasives - as well as two sanitizing methods, heat and chemicals. Chemical sanitizers must be at the proper concentration, temperature, and contact time to be effective against bacteria. Utensils require thorough cleaning, sanitizing, and drying to eliminate bacteria. Regular cleaning of the kitchen removes germs and uses a homemade solution of vinegar, water, and soap to disinfect surfaces.