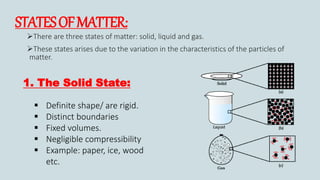
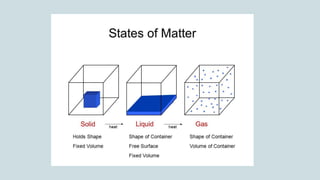
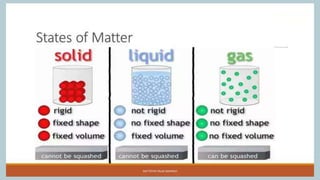
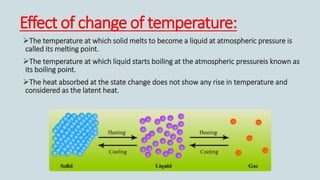
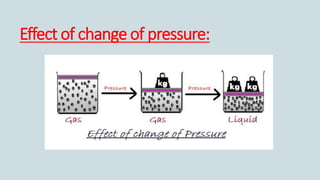
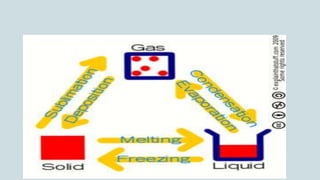
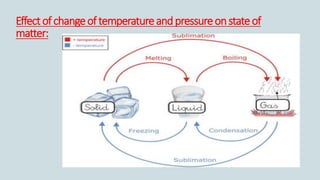
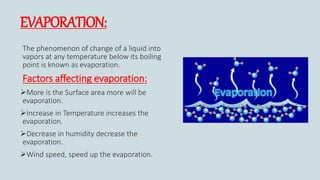
The document discusses the states of matter and how matter can change states. It introduces the three common states of matter as solid, liquid, and gas. Solids have a definite shape and volume while liquids have no definite shape but a fixed volume. Gases have no definite shape or volume. Matter can change states, such as water existing as ice, liquid water, and water vapor. The temperature at which a state change occurs depends on factors like melting point, boiling point, and the absorption of heat known as latent heat. Evaporation is defined as a liquid changing to a gas below the boiling point due to factors like surface area, temperature, humidity, and wind speed, which can cause cooling.