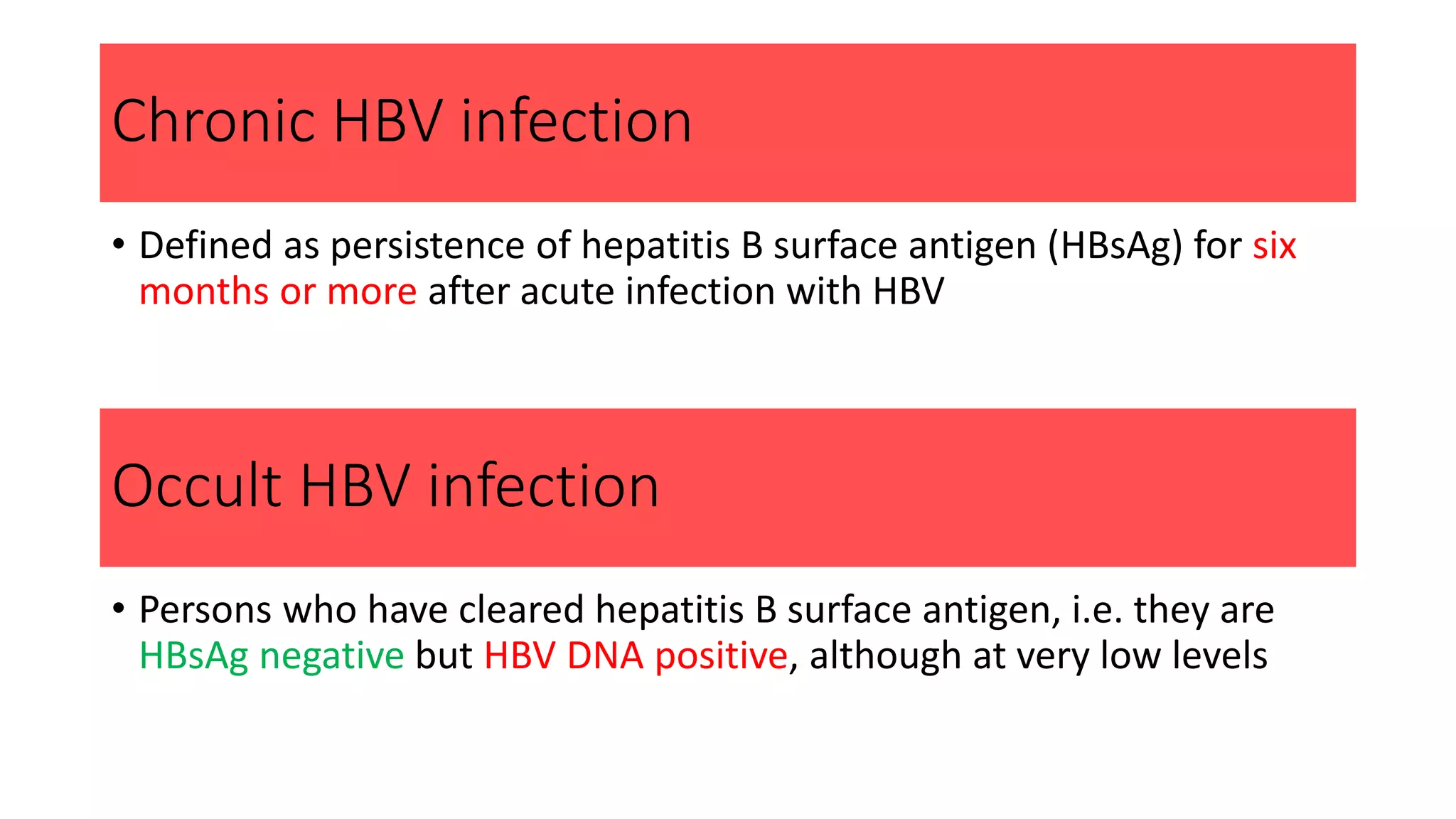
1. Chronic Hepatitis B (CHB) is caused by the HBV virus and can be either acute or chronic. It infects the liver and causes inflammation and necrosis.
2. Initial assessment of patients with CHB includes medical history, physical exam, liver disease markers and severity indicators, and testing of close contacts.
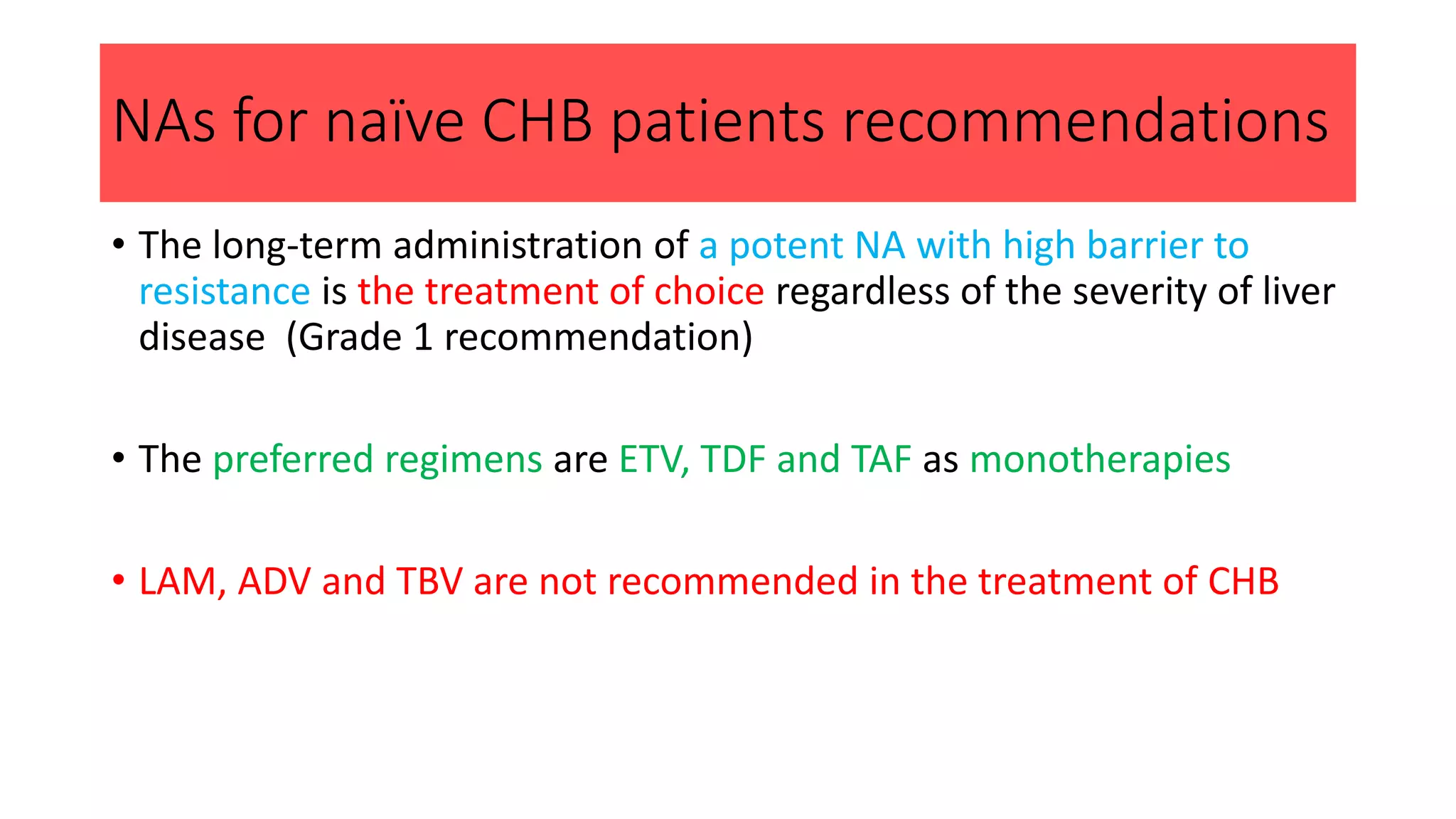
3. Treatment indications include elevated HBV DNA and ALT levels, cirrhosis, and family history of HCC. The preferred treatments are entecavir, tenofovir, and tenofovir alafenamide which have a high genetic barrier to resistance.