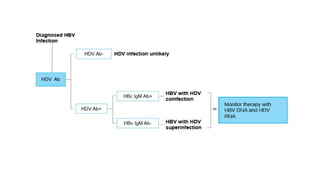
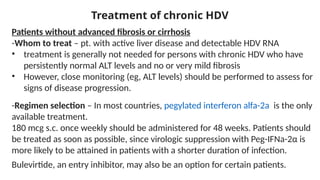
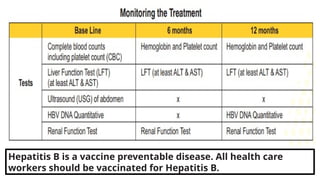
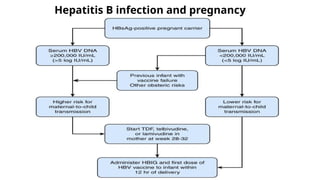
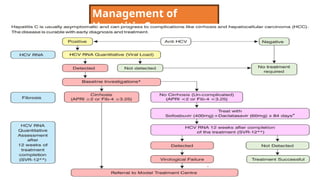
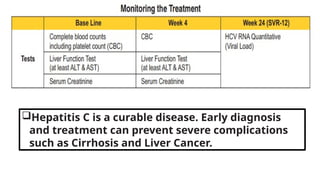
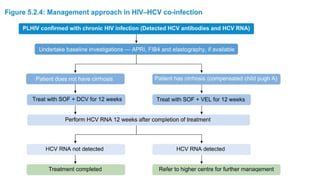
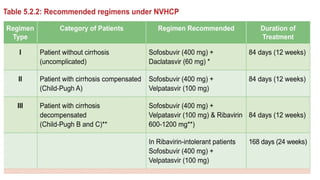
The document discusses the diagnosis and management of viral hepatitis, focusing on hepatitis B, C, D, and A. It highlights the significance of screenings, available treatments, and the importance of vaccination, particularly for hepatitis B. Additionally, it provides insights into co-infections, management strategies, and prevention measures for hepatitis D, underlining the public health implications of these viral infections.


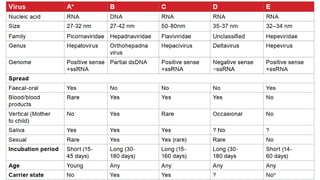
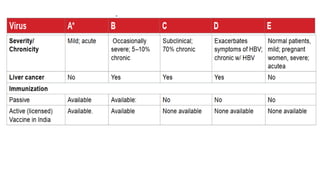


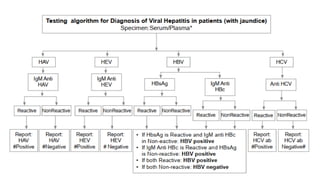

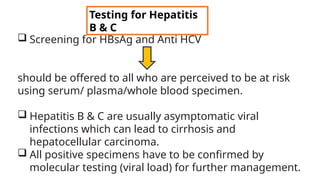
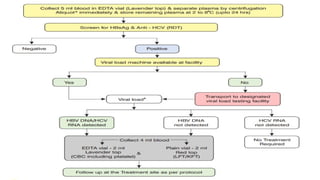
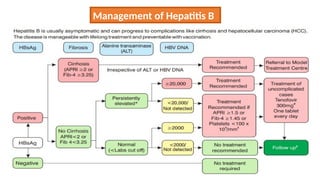







![Hepatitis A
presents with non-specific constitutional symptoms of low grade
fever, anorexia, nausea and vomiting, fatigue, malaise, arthralgia,
myalgia, headache, photophobia, pharyngitis, cough and coryza
may precede onset of jaundice by 1-2weeks.
Hepatitis E usually an acute self-limiting disease.
Acute hepatic failure- more likely in pregnancy and in malnourished state or
having pre-existing liver disease. Chronic HEV has been described almost
exclusively in immunocompromised patients.
Four genotypes (genotypes 1 to 4 [HEV1, HEV2, HEV3, and HEV4]).
Genotypes 1 and 2 more virulent, whereas genotypes 3 and 4 are
more attenuated and account for subclinical infections.
MANAGEMENT OF BOTH- USUALLY SUPPORTIVE](https://image.slidesharecdn.com/hepatitis-250120124804-23e10709/85/Hepatitissssssssssssssssssssssssssssssssss-29-320.jpg)