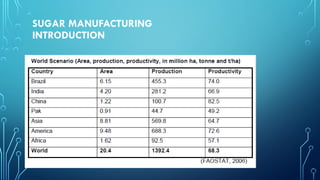
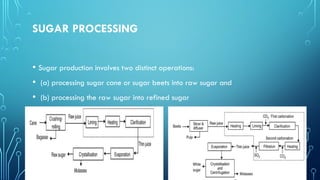
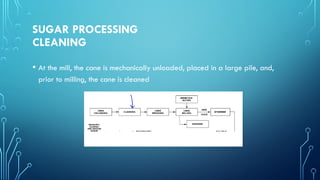
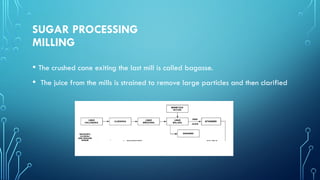
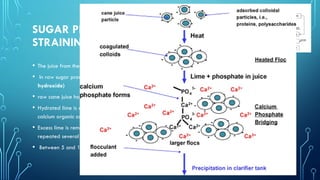
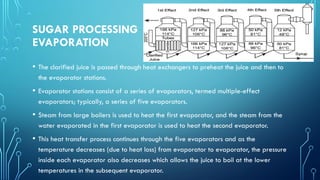
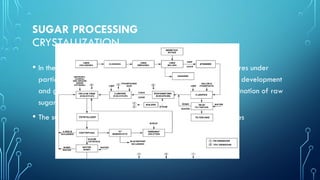
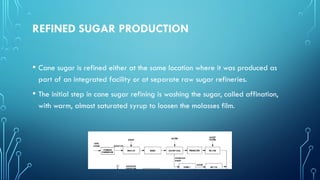
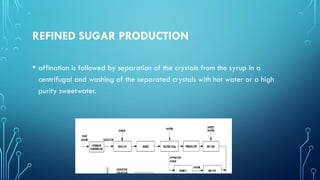
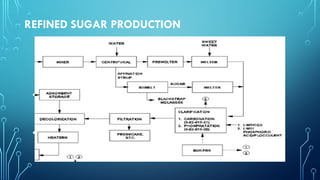
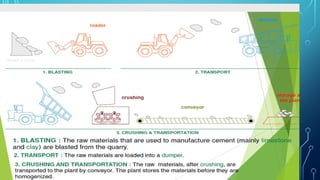
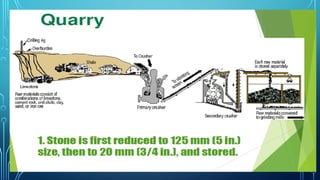
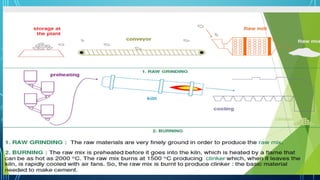
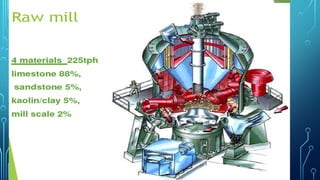
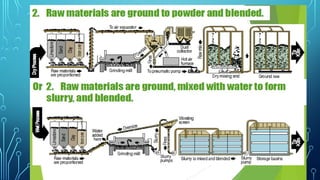
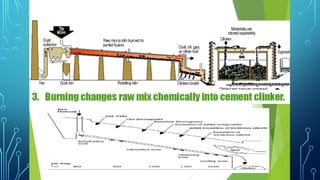
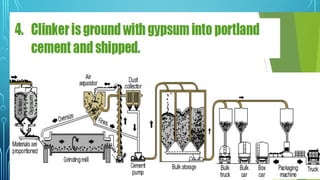
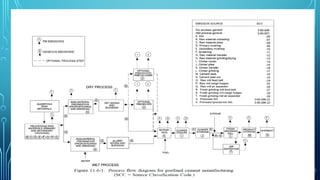
The document provides an overview of chemical process technology specifically in the sugar and cement industries. It details the sugar manufacturing process, including the various types of sugar cane, by-products, and processing steps such as milling, straining, evaporation, and crystallization. Additionally, it describes the composition and uses of cement, emphasizing hydraulic versus non-hydraulic types and their applications in construction.